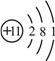��Ŀ����
����Ŀ��ij��ѧ��ȤС��Ϊ�˲ⶨ���ص�ʯ��ʯ����Ҫ�ɷ���CaCO3��������������������������ʵ�飺ȡ12����Ʒ�����ձ��У�����������120��һ������������ϡ���ᣨ���ʲ�����ˮҲ�����ᷴӦ�������������������ʣ������������ϵ��ͼ��ʾ�������ͼ�е����ݽ��м��㣺
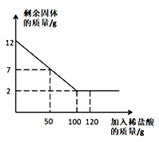
��1��ʯ��ʯ��Ʒ��CaCO3������Ϊ __________�ˣ�
��2�������������������������__________��������ݻ�ѧ����ʽд�������ļ��㲽�裩
���𰸡� 10 7.3%
����������1��������ٵ������Dzμӷ�Ӧ��̼��Ƶ�����������ʯ��ʯ��Ʒ��CaCO3������Ϊ��12g-2g=10g����2����ͼ����ǡ�÷�Ӧʱ����ϡ�����������100g����100g�������������ʵ�������x��
CaCO3 + 2HCl == CaCl2 + H2O + CO2��
100 73
10g x
![]() =
=![]() �����x=7.3g
�����x=7.3g
�����������������������![]() ��100%=7.3%
��100%=7.3%

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
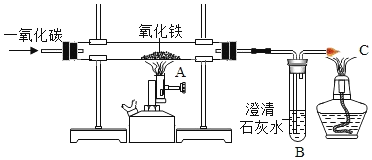
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�����Ŀ�������ģ�(1)����������ʵ���ѧʽ����������գ������к�����������________���������е�������________��������������ʱ���õ�һ�ּ_________����
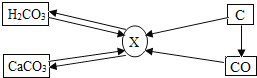
(2)���������ڿ�ݷ��������ǧ���������ͼʾ�ش�
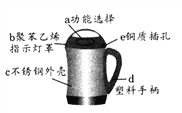
���������������ڽ�����Ͻ����_______(ѡ��һ�����ʱ��)�������л��ϳɲ��ϵ���_________(ѡ��һ�����ʱ��)�������ֱ�����������____________����(�����������������ȹ�����)��
�ڶ����ѳ�Ϊ�ڶ��ͥ�������Ʒ�������Ƕ�����һЩӪ���ɷֵ�ƽ������������������±��ش�
�ɷ� | ˮ | ������ | ֬�� | ���� | �� | �� | �� | ά����A |
��������/�� | 96.0 | 1.8 | 0.7 | 1.1 | 0.01 | 0.03 | 0.0005 | 0.015 |
�����к��е���Ԫ����__________��������ȱ��______Ԫ���������Ͳ����ڶ���������Ӫ�������ܹ�������������Ӫ������______________________��