��Ŀ����
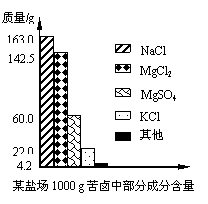
����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ��ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺��1����ʹ100g�ÿ�±�е�MgCl2��MgSO4��ȫת��ΪMg��OH��2��������Ҫ��ʯ�Ҷ��ٿˣ�
��2��������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵý���þ���ٿˣ��������������������������ܶ�Ϊ3.0g/L����

���𰸡���������1������״ͼ����֪��100g��±���Ȼ�þ������þ��������
���Ȼ�þ������þ�������������������Ʒ�Ӧ�Ļ�ѧ����ʽ���Լ�����ֱ���Ҫ�������Ƶ���������Ҫ�������Ƶ����������ֱ�����������þ������������������þ����������
��2����������þ������������ϡ���ᷴӦ�Ļ�ѧ����ʽ���Եõ��Ȼ�þ�������������õ�����þ������������������������ܶȿ��Եõ������������
����⣺��1��������״ͼ��֪100g��±���Ȼ�þ������Ϊ
142.5g×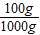 =14.25g
=14.25g
�轫�Ȼ�þת��Ϊ������þ��Ҫ�������Ƶ�����Ϊx������������þ������Ϊy��
MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2
95 74 58
14.25g x y
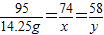 ��
��
��֮�ã�x=11.1g��y=8.7g
������״ͼ��֪100g��±������þ������Ϊ
60g× =6g
=6g
�轫����þת��Ϊ������þ��Ҫ�������Ƶ�����Ϊz������������þ������Ϊw��
MgSO4+Ca��OH��2=Mg��OH��2��+CaSO4
120 74 58
6g z w
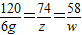
��֮�ã�z=3.7g��w=2.9g
����Ҫ������������Ϊ11.1g+3.7g=14.8g
�𣺹���Ҫ��ʯ��14.8��
��2��������������þ����������Ϊ
8.7g+2.9g=11.6g
�����ɽ���þ������Ϊm����������������Ϊn��
��Mg��OH��2+2HCl=MgCl2+2H2O��MgCl2 Mg+Cl2����
Mg+Cl2����
Mg��OH��2��MgCl2��Mg+Cl2��
58 24 71
11.6g m n
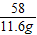 =
=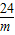 =
=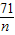 ��
��
��֮�ã�m=4.8g��n=14.2g
���������Ϊ=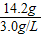 ��4.7L
��4.7L
�𣺿ɵý���þ4.8g������4.7L��
������������Ҫ�����йػ�ѧ����ʽ�ļ��㣬�ѶȽϴ������������Ҫ�Է�Ӧԭ������������Ҫ�Ը��ݷ���ʽ���м���IJ�����Ϥ��
���Ȼ�þ������þ�������������������Ʒ�Ӧ�Ļ�ѧ����ʽ���Լ�����ֱ���Ҫ�������Ƶ���������Ҫ�������Ƶ����������ֱ�����������þ������������������þ����������
��2����������þ������������ϡ���ᷴӦ�Ļ�ѧ����ʽ���Եõ��Ȼ�þ�������������õ�����þ������������������������ܶȿ��Եõ������������
����⣺��1��������״ͼ��֪100g��±���Ȼ�þ������Ϊ
142.5g×
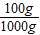 =14.25g
=14.25g�轫�Ȼ�þת��Ϊ������þ��Ҫ�������Ƶ�����Ϊx������������þ������Ϊy��
MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2
95 74 58
14.25g x y
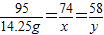 ��
����֮�ã�x=11.1g��y=8.7g
������״ͼ��֪100g��±������þ������Ϊ
60g×
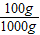 =6g
=6g�轫����þת��Ϊ������þ��Ҫ�������Ƶ�����Ϊz������������þ������Ϊw��
MgSO4+Ca��OH��2=Mg��OH��2��+CaSO4
120 74 58
6g z w
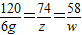
��֮�ã�z=3.7g��w=2.9g
����Ҫ������������Ϊ11.1g+3.7g=14.8g
�𣺹���Ҫ��ʯ��14.8��
��2��������������þ����������Ϊ
8.7g+2.9g=11.6g
�����ɽ���þ������Ϊm����������������Ϊn��
��Mg��OH��2+2HCl=MgCl2+2H2O��MgCl2
 Mg+Cl2����
Mg+Cl2����Mg��OH��2��MgCl2��Mg+Cl2��
58 24 71
11.6g m n
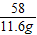 =
=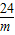 =
=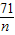 ��
����֮�ã�m=4.8g��n=14.2g
���������Ϊ=
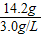 ��4.7L
��4.7L�𣺿ɵý���þ4.8g������4.7L��
������������Ҫ�����йػ�ѧ����ʽ�ļ��㣬�ѶȽϴ������������Ҫ�Է�Ӧԭ������������Ҫ�Ը��ݷ���ʽ���м���IJ�����Ϥ��

��ϰ��ϵ�д�
�����Ŀ
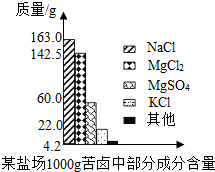 ����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ������ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺
����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ������ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺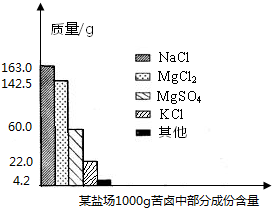 ����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ��ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺
����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ��ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺

 (2)������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵý���þ���ٿˣ��������������������������ܶ�Ϊ3.0 g/L����
(2)������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵý���þ���ٿˣ��������������������������ܶ�Ϊ3.0 g/L����