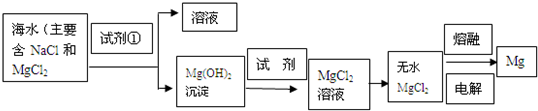
��Ŀ����
����Լռ����������71%������ʮ�־�
�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ������
��±�����Ƶý���þ�Ȼ���ԭ�ϡ���ͼ������ij��
����±�в��ֳɷֺ�������ͼ���Լ��㣺
(1) ��ʹ100 g�ÿ�±�е�MgCl2��MgSO4��ȫת��ΪMg(OH)2��������Ҫ20% NaOH��Һ���ٿˣ�
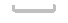 (2)������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵý���þ���ٿˣ��������������������������ܶ�Ϊ3.0 g/L����
(2)������������ȫת��Ϊ��ˮMgCl2����������״̬�½��е�⣬�ɵý���þ���ٿˣ��������������������������ܶ�Ϊ3.0 g/L����
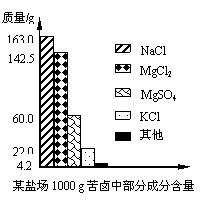
�⣺��1����100 g��±��MgCl2��MgSO4��ȫת��ΪMg(OH)2����NaOH�������ֱ�Ϊx��y��
MgCl2 + 2NaOH = Mg(OH)2��+2 NaCl
95 80
14.25g x
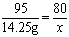 x = 12 g
x = 12 g
MgSO4 + 2 NaOH = Mg(OH)2��+ Na2SO4
120 80
6g y
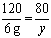 y = 4 g
y = 4 g
NaOH��Һ������Ϊ�� 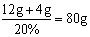
��2���ɵ�Mg����Ϊ�� 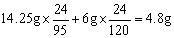
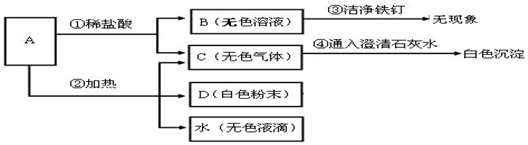
����Cl2�������� 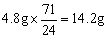
�����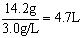

��ϰ��ϵ�д�
�����Ŀ
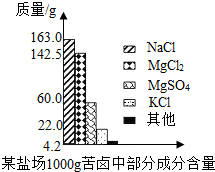 ����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ������ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺
����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ������ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺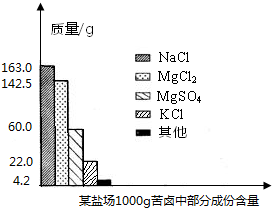 ����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ��ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺
����Լռ����������71%������ʮ�־�Ŀ���DZ������±�Ǻ�ˮ��ȡʳ�κ�IJ�Һ�����ÿ�±�����Ƶý���þ�Ȼ���ԭ�ϣ���ͼ��ij�γ���±�в��ֳɷֺ�������ͼ���Լ��㣺