��Ŀ����
ij��Ĵ����ձ黼��һ����ֵļ������ѷ���ʧ���������������п�Ժר�ҶԸõ���ˮԴ��ֲ�����������ԭ���Ǹ�����ɽ�����IJɣ�ʹˮԴ�к��裨Ti��Ԫ�ع������£�
��1���ߴ���������뵼����ϣ��۸���ƽ��൱��д��������ʹ��������ת��Ϊ����Ļ�ѧ����ʽ������֪��Ba��OH��2Ϊǿ�������ˮ��BaSO4������ˮ��
Ti2SO4����Һ����Ti��OH������Һ�������ɡ�Ti2O��Ti
�� �� ��
��2��Tiԭ�ӽṹʾ���ͼΪ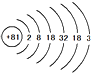 ����ԭ�ӵ����ԭ������Ӧ ������ڡ��������ڡ���С�ڡ���81�����������Ϊ ��
����ԭ�ӵ����ԭ������Ӧ ������ڡ��������ڡ���С�ڡ���81�����������Ϊ ��
��1���ߴ���������뵼����ϣ��۸���ƽ��൱��д��������ʹ��������ת��Ϊ����Ļ�ѧ����ʽ������֪��Ba��OH��2Ϊǿ�������ˮ��BaSO4������ˮ��
Ti2SO4����Һ����Ti��OH������Һ�������ɡ�Ti2O��Ti
��2��Tiԭ�ӽṹʾ���ͼΪ
 ����ԭ�ӵ����ԭ������Ӧ
����ԭ�ӵ����ԭ������Ӧ���㣺���ʵ��ת�����Ʊ�,ԭ�ӽṹʾ��ͼ�����ӽṹʾ��ͼ,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺���ʵ��Ʊ�,��ѧ����������غ㶨��
��������1����������������ȡ����ķ�Ӧд����Ӧ�Ļ�ѧ����ʽ��
��2���������ԭ�����������������������Ĺ�ϵ��Ԫ�ص�������ԭ�ӽṹ�Ĺ�ϵ�����ش�
��2���������ԭ�����������������������Ĺ�ϵ��Ԫ�ص�������ԭ�ӽṹ�Ĺ�ϵ�����ش�
����⣺��1���������֪��ʹ��������ת��Ϊ����Ļ�ѧ����ʽ�У�Ti2SO4+Ba��OH��2=BaSO4��+2TiOH��2TiOH
Ti2O+H2O��Ti2O+H2
2Ti+H2O��
��2���������ԭ�������������������������ĺͣ�Ti����������81������������0�����ԣ���ԭ�ӵ����ԭ������Ӧ����81����������������3���ڻ�ѧ��Ӧ����ʧȥ3�����ӣ����������Ϊ+3��
�ʴ�Ϊ����1����Ti2SO4+Ba��OH��2=BaSO4��+2TiOH��2TiOH
Ti2O+H2O��Ti2O+H2
2Ti+H2O��
��2�����ڣ�+3��
| ||
| ||
��2���������ԭ�������������������������ĺͣ�Ti����������81������������0�����ԣ���ԭ�ӵ����ԭ������Ӧ����81����������������3���ڻ�ѧ��Ӧ����ʧȥ3�����ӣ����������Ϊ+3��
�ʴ�Ϊ����1����Ti2SO4+Ba��OH��2=BaSO4��+2TiOH��2TiOH
| ||
| ||
��2�����ڣ�+3��
������������Ҫ�����˻�ѧ����ʽ����д������ṹ�����壬��ѧ����ʽ������Ҫ�Ļ�ѧ���Ӧ��ǿѧϰ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��������������κ�ˮ�ķ�Ӧ�������кͷ�Ӧ�����ڹ�ũҵ�������ճ��������й㷺����;������Ӧ��һ�����кͷ�Ӧԭ���ص��ǣ�������
| A��ʩ����ʯ�Ҹ����������� |
| B����ϡ��ˮ�����ó涣ҧ |
| C������ʯ�Һ�����ͭ���Ʋ�����Һ |
| D����NaOH��Һϴ��ʯ�Ͳ�Ʒ�еIJ������� |
���и��������У�ǰ�����ڴ�����������ڻ������ǣ�������
| A������ Һ�� |
| B����ˮ����� ��Ȫˮ |
| C����ˮ ��ˮ |
| D��������Ŀ��� ����Ⱦ�Ŀ��� |