��Ŀ����
2011�����������ĸ���Cr����Ⱦ�¼���˵��������������Һ���������ŷŶ��������滷���м����Σ������ⷨ�Ǵ�������Ⱦ��һ�ַ������䴦�����̷����ķ�Ӧ�ɱ�ʾΪ��
6Fe+K2Cr2O7+H2SO4+17H2O=6Fe��OH��3��+2Cr��OH��3��+6X+K2SO4
��1��K2Cr2O7��CrԪ�صĻ��ϼ�Ϊ ��
��2������X�Ļ�ѧʽΪ ��
��3��ʵ�����ռ�Xʱ�����������ſ���������������X ���������ʣ�
6Fe+K2Cr2O7+H2SO4+17H2O=6Fe��OH��3��+2Cr��OH��3��+6X+K2SO4
��1��K2Cr2O7��CrԪ�صĻ��ϼ�Ϊ
��2������X�Ļ�ѧʽΪ
��3��ʵ�����ռ�Xʱ�����������ſ���������������X
���㣺�й�Ԫ�ػ��ϼ۵ļ���,����������ռ�����,�����غ㶨�ɼ���Ӧ��
ר�⣺��ѧʽ�ļ���,���ʵı仯������,��ѧ����������غ㶨��
��������1�����ݻ�������Ԫ�ػ��ϼ۴�����Ϊ�㣬���K2Cr2O7�Ļ�ѧʽ���н���⣮
��2�����������غ㶨�ɽ��з�����
��3������������Ϣ���з�����
��2�����������غ㶨�ɽ��з�����
��3������������Ϣ���з�����
����⣺��1����Ԫ����+1�ۣ���Ԫ����-2�ۣ����Ԫ�صĻ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬�ɵã���+1����2+2x+��-2����7=0����x=+6�ۣ�
��2���ɷ�Ӧ�Ļ�ѧ����ʽ6Fe+K2Cr2O7+H2SO4+17H2O=6Fe��OH��3��+2Cr��OH��3��+6X+K2SO4����Ӧǰ���Ԫ��ԭ�ӵĸ���Ϊ
��Ӧǰ ��Ӧ��
Feԭ�� 6 6
Kԭ�� 2 2
Sԭ�� 1 1
Oԭ�� 28 28
Crԭ�� 2 2
Hԭ�� 36 24
���ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬���ж�����X��6�������к���12��Hԭ�ӣ�����X�Ļ�ѧʽ�ɱ�ʾΪH2��
��3��ʵ�����ռ�����ʱ�����������ſ����������������������ܶȱȿ���С���������ʣ�
�����1��+6����2��H2����3���ܶȱȿ���С��
��2���ɷ�Ӧ�Ļ�ѧ����ʽ6Fe+K2Cr2O7+H2SO4+17H2O=6Fe��OH��3��+2Cr��OH��3��+6X+K2SO4����Ӧǰ���Ԫ��ԭ�ӵĸ���Ϊ
��Ӧǰ ��Ӧ��
Feԭ�� 6 6
Kԭ�� 2 2
Sԭ�� 1 1
Oԭ�� 28 28
Crԭ�� 2 2
Hԭ�� 36 24
���ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬���ж�����X��6�������к���12��Hԭ�ӣ�����X�Ļ�ѧʽ�ɱ�ʾΪH2��
��3��ʵ�����ռ�����ʱ�����������ſ����������������������ܶȱȿ���С���������ʣ�
�����1��+6����2��H2����3���ܶȱȿ���С��
�����������ѶȲ����������û��ϼ۵�ԭ�����ָ��Ԫ�صĻ��ϼ۵ķ��������������غ㶨���ƶ����ʻ�ѧʽ�ķ����ȼ��ɽ���⣮

��ϰ��ϵ�д�
�����Ŀ
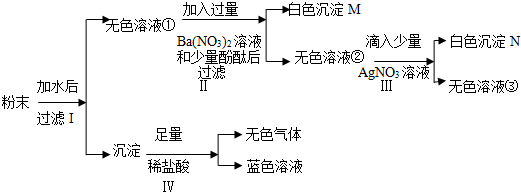
��ͼʾʵ���������������ǣ�������
A��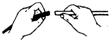 |
B��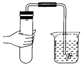 |
C��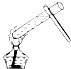 |
D��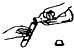 |
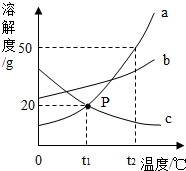 ��ͼ�����ֹ�������a��b��c���ܽ�����ߣ�
��ͼ�����ֹ�������a��b��c���ܽ�����ߣ�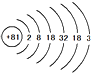 ����ԭ�ӵ����ԭ������Ӧ
����ԭ�ӵ����ԭ������Ӧ