��Ŀ����
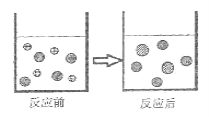
����Ŀ��ѧУ��ѧʵ��С��ͬѧ̽�����ᡢ���������������ʵĻ�ѧ���ʣ�ȡ8֧�Թܷքe��A��H��ź���������ʵ�顣

��1��ʵ���й۲쵽�����ݳ��ֵ��Թ���_____���г������ɵ��Թ���_____��
��2��ʵ��������������������Թ��з�����Ӧ�Ļ�ѧ����ʽΪ_____��
��3��ʵ���ij�Թ���Ϊ��ɫ��Һ�������м��������_____����Һ��Ϊ��ɫ���ɴ��ƶϣ����Թ������ʢ�е�������_____��
��4��ʵ���ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬһ��ʱ��������ݳ��֣������Һ�д��ڵ����ֵ�����Ϊ_____��
���𰸡�CD G H Ca��OH��2+2HCl��CaCl2+2H2O NaOH��Һ�������𰸾��ɣ� ��ɫʯ����Һ NaOH��Na2CO3
��������
��1��ʵ���й۲쵽�����ݳ��ֵ��Թ��ǣ�CD����ΪC����̼��ƺ����������Ȼ��ơ�ˮ�Ͷ�����̼��D����̼���ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��G��̼�������������Ʒ�Ӧ����̼��ư�ɫ�������������ƣ�H�ж�����̼��ʹ�����ʯ��ˮ����ǣ��ʴ�Ϊ��CD�� G H��
��2��ʵ��������������������Թ���F�����������������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ����ƽ���ɣ��ʴ�Ϊ��Ca��OH��2+2HCl��CaCl2+2H2O��
��3��������ɫʯ����Һ�����Ժ�ɫ����������ɫ������ʵ���ij�Թ���Ϊ��ɫ��Һ���������м��������NaOH��Һ����Һ��Ϊ��ɫ���ɴ��ƶϣ����Թ������ʢ�е���������ɫʯ����Һ���ʴ�Ϊ��NaOH��Һ�������𰸾��ɣ�����ɫʯ����Һ��
��4��ʵ���ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬһ��ʱ��������ݳ��֣������Һ�е�������NaOH��Na2CO3���ȷ����кͷ�Ӧ������̼���Ʒ�Ӧ������ð�����ʴ�Ϊ��NaOH��Na2CO3��

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�����Ŀ����5�֣�KNO3��NaCl�ڲ�ͬ�¶�ʱ���ܽ�����±���ʾ����ش��������⣺
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | |||||||||
�ܽ��/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 | ||||||||
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 | |||||||||
��1�������ϱ����ݣ����Ƴ�KNO3��NaCl���ܽ��������ͼ��ʾ��
ͼ���ܱ�ʾKNO3�ܽ�����ߵ��� ���A����B����

��2���ɱ������ݷ�����֪��KNO3��NaCl��ijһ�¶�ʱ������ͬ���ܽ� �ȣ����¶ȵķ�Χ�� ��
��3��ij��ȤС������һ��ʵ�飺

����ʵ������еõ�����Һһ�����ڲ�������Һ���� ����������ţ�������������ȴ��10�������ˣ����ɻ��յõ�KNO3���� g��
��4��������к��������Ȼ���ʱ����ͨ�� ��������ᾧ�����½ᾧ�����ķ����ᴿ��
����Ŀ��������ӹ��ɵ������������һ��������ҩ�ġ�����Ʒ���ɽ������г��ϳ�����һЩ��ð��������������������۹۲��ѱ���٣�Ϊ�����������ṩ���������ҳ����������������������ϵIJ��죮Ϊ�ˣ�ij���̾��ṩ���桢�������������Ʒ���й��������۵����������
Ʒ�� | ��� | �ɷ� | �������� |
������� | ��ɫ��ζ��ĩ | ��ҪΪ̼��ƣ����ܺ�����̼��þ���ǽǵ��ȣ� | ֱ�ӽ�����ĥ�ɷ�ĩ�� |
������� | ��ɫ��ζ��ĩ | ���Ƿۣ���Ҫ�ɷ�ΪCaCO3�������ܺ�����̼��þ�������������ۣ� | ������Ư�ף�Ư�������õ���NaOH����Ȼ��ĥ�ɷ�ĩ�� |
���У���������еĿǽǵ��ף������ʵ�һ�֣�������ˮ������Ũ������Ⱥ����ɫ��ij�о���ѧϰС����ṩ���������۽���������̽����
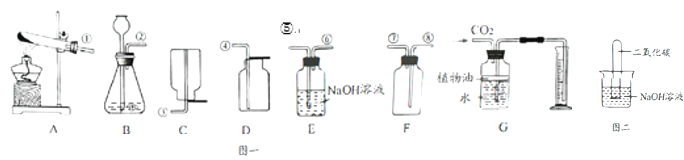
��̽��һ�����������۵ij���������
��1���桢������۾�����_____���������������������������ȡ�����������Ʒ���Թ��У�����_____�����ȣ����۲쵽���ɫ�������Ʒ��������ۣ�
��2����ȡ��������������������Թ��У���������ˮ���ã����ϲ���Һ�ֱ���2֧�Թ��У���һ֧�Թ��еμ�_____�����Һ�����ɫ��˵�����в��е��ۣ�������һ֧�Թ��м���1��2��_____����Һ�����ɫ��˵����Ư�ױ��ǵĹ������õ����������ƣ�ʹ��Һ�ʼ��ԣ�
��̽�������ⶨ�����������̼��Ƶ�����������
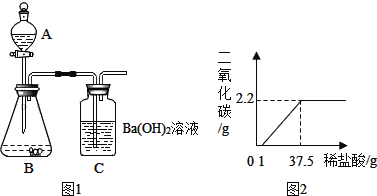
������������������ͼ1��ʾʵ��װ�ã�ijѧ����סCĩ�˲������ܿڣ���A��װ������ˮ��ȡ���ϲ����ӣ�����������A��ˮ����ȫ�����£�����Ϊ��װ���Ƿ�©����_____������©����������©������
�����ȷ��ȡ5.00g�������Ʒװ������B�У���A��װ��һ��Ũ�ȵ�ϡ���ᣮ
�������B���������Ʒ�еμ�������ϡ���ᣮ������B��C�пɹ۲쵽������ֱ�Ϊ_____��_____��
���������ȫ��Ӧ��C�еĻ��Һ��_____��_____������Ƶð�ɫ���������Ϊ9.85g��������װ����ԭ�п�����װ���������Ӱ�죩���ɴ�����������Ʒ��̼��Ƶ���������Ϊ100%��
��ʵ�鷴˼������ⶨ������о�С���ͬѧ�Ǹе��������⣬�������۷����������Ϊ���ܵ�ԭ����_____������������ţ�
����Ʒ�к���̼��þ ������δ�μ�����
��CO2�����ٶ�̫�쵼��δ��Ba��OH��2��ȫ���� ��װ��B��ˮ������HCl�Ƚ���װ��C��
����չ���죩ͬѧ�Ǹ��ݲⶨ���ݼ������Ʒ��̼������ӵ���������Ϊ_____%��
��ͬѧ�����̽�����в���Ҫװ��C��ֻҪ�õ�����ƽȷ����װ��A��B��Ӧǰ�����������Ҳ��������������Ʒ��̼������ӵ���������������Ϊ������õ�̼������ӵ���������������������ֵ��ȣ�����_____����������������ƫ��������ƫС������������ͬѧ�Ǿ�����ȷ�ⶨ���õ�����ϡ����������Ķ�����̼����������ϵ��ͼ2��ʾ��
���������ۣ�
��1���ڼ���ϡ����Ĺ����У���ʼû���������������Ϊ������Ʒ�л���������_____������̼��þ��������������������Ե�ʣ�
��2������ͼʾ���������ϡ�����������������____�������м�����̣�