��Ŀ����
����Ŀ��ij�����ڵ�ȼ�������·�����Ӧ��������ͷ�Ӧ�ﹲ���֣����ǵ���ʾ��ͼ�ͷ�Ӧǰ����������±���ʾ��
������� | �� | �� | �� | �� |
|
��ʾ��ͼ |
|
|
|
| |
��Ӧǰ����/g | 68 | 100 | 1 | 0 | |
��Ӧ������/g | 0 | x | y | z |
(1)��![]() ���ɵ�������
���ɵ�������![]() ��������Ԫ�صĻ��ϼ�Ϊ_____��
��������Ԫ�صĻ��ϼ�Ϊ_____��
(2)���е����������У��������������_________ (�ѧʽ��������������________������ţ��ٻ�����ڵ��ʡ��۴�����ܻ�����
(3) ��ͼ˵����ѧ��Ӧǰ�����ı������______����������������ԭ��������
������Ӧ�Ļ�ѧ����ʽΪ____________________________��
(4)һλͬѧ�ڼ���x��y��z��ֵ�Ĺ����У��г������µ�ʽ��������ȷ����______������ĸ��ţ���
A. x + y +x = 169 B. y + z = 168 C. (100-x)��z = 32��64 D.(l00-x)��(y-1) = 8��3
���𰸡� -2 H2O��SO2 �ڢ� ���� 2H2S+3O2![]() 2H2O+2SO2 AD
2H2O+2SO2 AD
�����������������غ㶨��֪��(1)���������ܼ۴�����Ϊ��ԭ����ͨ��Ϊ-2�ۣ���![]() ���ɵ�������
���ɵ�������![]() ��������Ԫ�صĻ��ϼ�Ϊ��x���v-2�w��2��0,x��+4��(2)���е����������У��������������H2O��SO2������������������Ԫ����ɵĻ��������һ��Ԫ������Ԫ�أ����������ڢڵ��ʡ��۴������������ͬ��Ԫ����ɵĴ����(3) ��ѧ��Ӧǰ�����ı�����Ƿ��ӣ���ѧ�仯��ʵ���Ƿ��ӵĻ��֣�ԭ�ӵ�������ϣ���Ӧ�Ļ�ѧ����ʽΪ��2H2S+3O2
��������Ԫ�صĻ��ϼ�Ϊ��x���v-2�w��2��0,x��+4��(2)���е����������У��������������H2O��SO2������������������Ԫ����ɵĻ��������һ��Ԫ������Ԫ�أ����������ڢڵ��ʡ��۴������������ͬ��Ԫ����ɵĴ����(3) ��ѧ��Ӧǰ�����ı�����Ƿ��ӣ���ѧ�仯��ʵ���Ƿ��ӵĻ��֣�ԭ�ӵ�������ϣ���Ӧ�Ļ�ѧ����ʽΪ��2H2S+3O2![]() 2H2O+2SO2 ��(4)һλͬѧ�ڼ���x��y��z��ֵ�Ĺ����У��г������µ�ʽ��������ȷ���ǡ�
2H2O+2SO2 ��(4)һλͬѧ�ڼ���x��y��z��ֵ�Ĺ����У��г������µ�ʽ��������ȷ���ǡ�
�ף��� �� ������ 2H2S��3O2 ![]() 2H2O��2SO2
2H2O��2SO2
68 100-x y-1 z 68 96 36 128
68��100-x��y-1��z, x + y +z= 169, y +z= 101, (l00-x)��(y-1) =96��36 ��8��3,
(100-x)��z = 96��128��3��4.����ѡAD��
�㾦�����Ӧ�������غ㶨������ɱ�����Ҫһ����

 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
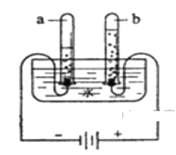
�ο�����������100��ϵ�д�����Ŀ��С����ͨ��ʵ��֤�������������ǹ�������ֽ�Ĵ�������һ���⡣����Ʋ�������±���ʾ��̽��ʵ�飺
ʵ��һ��ȡ5%�Ĺ���������Һ���Թ��У���������ǵ�ľ���������ݲ�����ľ������ȼ��
ʵ�������ʢˮ���Թ��м���������̣����������ľ���������ݲ�����
ʵ������________________����������ǵ�ľ�����д������ݲ�����ľ����ȼ��
���ۣ����������ǹ�������ֽ�Ĵ�����
(1)�����С��ͬѧ��дʵ�����IJ���_______________��
(2)ʵ�����Ŀ����____________________��
(3)С��ͬѧ��Ϊ��������ʵ�黹������ȫ�ó����ۣ�������_________��
(4)Сΰͨ�������Ϸ��ֹ�������ֽ�����ö��������⣬��������ͭ(CuO)��������������
��������⡿����ͭ�Ƿ�ȶ������̵Ĵ�Ч�����ã�Ӱ������طֽ����ʵ���������Щ�أ�
�����ʵ�顿�����ɵ����������Ϊ������������л���ʵ��(��������Ӱ��ʵ������ؾ�����)��
ʵ����� | KClO3������ | �������ʵ����� | �¶� | ��������� | ��Ӧ����ʱ�� |
һ | 2.0g | / | 3300C | 100mL | t1 |
�� | 2.0g | CuO 0.5g | 3300C | 100mL | t2 |
�� | 2.0g | MnO2 0.5g | 3300C | 100mL | t3 |
�� | 2.0g | MnO2 | 3800C | 100mL | t4 |
����t1__________t2(��������������������С����)��˵������ͭ�ܼӿ�����صķֽ����ʡ�
�ڱȽ�����ͭ�Ͷ������̴�Ч����ʵ������________________��
��д��ʵ������漰��Ӧ�ķ��ű���ʽ_______________��
�ܽ�ʵ������ʵ���ĶԱȣ��ɵó���ѧ��Ӧ�������¶ȵĹ�ϵ����ôʵ�����п���MnO2������ӦΪ_______g����t3��t4����ѧ��Ӧ�������¶ȵĹ�ϵ��______��