��Ŀ����
����Ŀ����ʵġ�̼�������˵����������һ���߽���̼�������硣
�ٵ���̼�ڳ����¾����ȶ��ԣ�����___________���ʡ������������ʻ�ѧ���ʣ�
��ľ̿���л�ԭ�ԣ�����ұ�����������ڡ���������ƽ���ϵ����
��Fe3O4+��C![]() ��Fe+��CO2��____________
��Fe+��CO2��____________
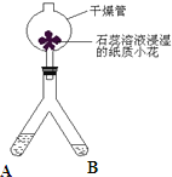
�۽����㽭��ѧʵ�����ﵮ��������������IJ��ϡ�����̼���ࡱ����̼Ԫ����ɣ����ж�ṹ�����Ժá�����ʯ���к�ǿ����������������ˮ�����������ʯ�ͼ������Կɻָ�ԭ״������̼�����˵����ȷ����_______������ĸ��ţ���
A.���������� B.���ظ����� C.���Դ�������ʯ��й©
����Ȼ����ʯ�������̼����ơ�Ca(HCO3)2���γ��ܶ���̼�������_______��Ԫ����ɣ�������Ԫ������Ԫ�ص����ʵ���֮����________��0.5mol�������и�Ԫ�ص�������_____g��0.5mol��������Լ����________����ԭ�ӡ�
���𰸡� ��ѧ���� 1,2,3,2 ABC 4 1:3 20 6.02��1023
���������٢ٵ���̼�ڳ����¾����ȶ��ԣ����ڻ�ѧ�������ڻ�ѧ����ʽ��ƽΪ��2C+ Fe3O4![]() 3Fe+2CO2������A��̼���࣬���ж�ṹ�����Ժá�����ʯ���к�ǿ����������������ˮ������ȷ��B������̼���ཫ�����ʯ�ͼ������Կɻָ�ԭ״�����ظ�ʹ�ã���ȷ��C������̼�����������ԣ���ʯ���к�ǿ�������������ɴ�������ʯ��й©����ȷ����̼����Ƶ�һ�������й���11��ԭ�ӣ�������Ԫ������Ԫ�ص����ʵ���֮����2:6=1:3��0.5mol�������и�Ԫ�ص�������40g/mol��0.5mol=20g��0.5mol��������Լ����0.5mol��2��6.02��1023=6.02��1023��̼ԭ�ӡ�
3Fe+2CO2������A��̼���࣬���ж�ṹ�����Ժá�����ʯ���к�ǿ����������������ˮ������ȷ��B������̼���ཫ�����ʯ�ͼ������Կɻָ�ԭ״�����ظ�ʹ�ã���ȷ��C������̼�����������ԣ���ʯ���к�ǿ�������������ɴ�������ʯ��й©����ȷ����̼����Ƶ�һ�������й���11��ԭ�ӣ�������Ԫ������Ԫ�ص����ʵ���֮����2:6=1:3��0.5mol�������и�Ԫ�ص�������40g/mol��0.5mol=20g��0.5mol��������Լ����0.5mol��2��6.02��1023=6.02��1023��̼ԭ�ӡ�

����Ŀ��ʵ���Ҳ���װ������ͼ��ʾ����ش��������⡣

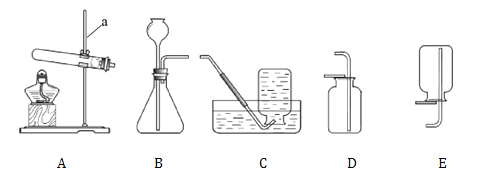
��1��ʵ���ҿ��������·����Ʊ�һЩ���������壬����ͼ1����ĸ�����д�±��հס�
�������� | ��ȡ������ | ����װ�� | �ռ�װ�� |
�ټ��ȸ������ | O2 | A | _____��F |
�ڴ���ʯ��ϡ������ | CO2 | C | _____ |
�ۼ��ȶ������̺�Ũ���� | Cl2 | _______ | D |
�v2�w��װ��A��ȡO2ʱ,װ��ҩƷǰӦ���е�ʵ�������_________��ѡ��Fװ���ռ�O2ʱ������ʵ���������ȷ���� ________________������ţ���
�ٵ�ȼ�ƾ���ǰ��������ƿע��ˮ���ò���Ƭ��סƿ�ڣ�������ʢˮ��ˮ���У�
�ڼ���ʱ�������������ݴӵ��ܿ�ð�������������ܿ����뼯��ƿ���ռ����壻
�ۼ����������ˮ���ò���Ƭ��סƿ�ڣ��ٽ�����ƿ�Ƴ�ˮ�ۣ����������ϣ�
�v3�w��������������ȡCO2ʱ������Ӧ�Ļ�ѧ����ʽΪ____________________������ͼ2��װ�ü���CO2���壬������Ӧ��___���a����b������ͨ�롣
��4����������������ȡ���ռ�����Cl2�������������е����������г�������������ˮ�����⣬����_���ѧʽ����