题目内容
【题目】用化学方法改造物质——“二氧化碳变汽油”。
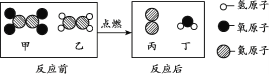
二氧化碳是化石燃料燃烧的产物,汽油(主要成分为含有5个-11个碳原子的碳氢化合物)是全球用量最大的液体燃料.如果有人告诉你“二氧化碳能变成汽油”,你相信吗?近日,中科院大连化学物理研究所研制出一种新型多功能复合催化剂,通过如图示意的I、II、III三个环节,将二氧化碳成功转化为汽油.(图中a、b是两种起始反应物的分子结构模型,c、d是最终制得的汽油中所含物质的分子结构模型)
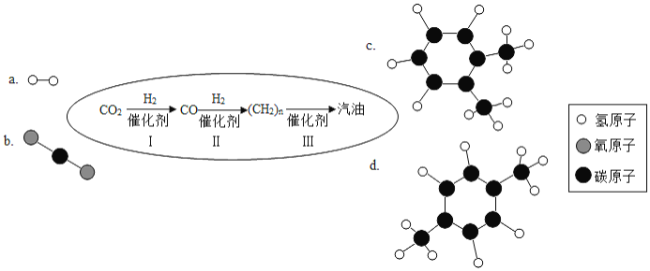
回答问题:
(1)催化剂能实现物质间的高效转化。下列有关催化剂的叙述正确的是__________ (填标号)。
A.催化剂可以改变化学反应速率
B.化学反应前后催化剂的质量不变
C.化学反应前后催化剂的化学性质发生改变
(2)在一定温度、压强及催化剂的条件下,环节I还生成了一种相对分子质量最小的氧化物,写出化学方程式:___________。
(3)观察c和d的分子结构模型,二者是否为相同物质? _______ (填“是”或“否”)。写出c的化学式:_______。
(4)“二氧化碳变汽油”的成果使我国成为此领域的领跑者。你认为该成果可解决的问题是__________、________ (答出两条即可)。
【答案】A、B CO2+H2![]() CO+H2O 否 C8H10 降低大气中的二氧化碳含量 缓解能源短缺
CO+H2O 否 C8H10 降低大气中的二氧化碳含量 缓解能源短缺
【解析】
(1)催化剂在化学反应中可以改变反应的速率,而其本身的质量与化学性质在反应前后保持不变;
(2)在一定温度、一定压强和催化剂存在的条件下,环节I除生成CO外,还生成了一种化合物水,对应的化学方程式为:CO2+H2![]() CO+H2O;
CO+H2O;
(3)认真观察c和d两种物质的分子结构模型,试写出cd物质的化学式都是C8H10,c和d的化学式相同但不属于同种物质;
(4)“二氧化碳变汽油”的研究成果,使我国成为此领域的世界领跑者;该成果的现实意义是有效降低CO2造成的温室效应,减少对传统化石能源的依赖。