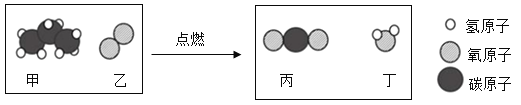
��Ŀ����
����Ŀ�������������������й㷺Ӧ�á�
(1) 2018�꣬�����������ĸ�����ͨ�����ǵij��н����ӷ��㡢��ݣ�����������Ҫ�����Ľ������ϡ�������·��ʹ�úܶ��ͭ�ߣ�����������ͭ����չ�Ժ�_______________________��������������������⣬��Ҫ��Ϊ�˷�ֹ������⣬������ԭ����__________________����������Դ��;������ֹ�����ĸ�ʴ�⣬����____________________ (��һ��)��

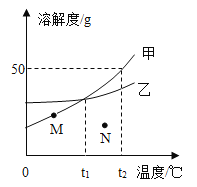
(2)�����ֶ���_________________�Ͻ������к�̼���ȸ���_________________ (��ߡ��͡�)��
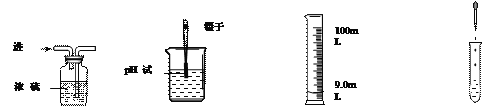
(3)��AgNO3��Һ�м���һ�������������Ļ�Ϸ�ĩ����ַ�Ӧ����ˣ��õ�������dz��ɫ��Һ�����ڸ���������Һ����������˵��:
���������м���ϡ���ᣬһ�������ݲ���
������Һ�м���ϡ���ᣬһ���г�������
����Һ��һ������Fe(NO3)2,���ܺ���AgNO3
��������һ��û���������ܺ�����
����˵������ȷ���� _____________��
���𰸡������� ����������ˮ���� �������պ����� �� �� ��
��������
��1����ͭ�����ߣ�������ͭ����չ�Ժ͵����ԡ��������������ֹ�����ԭ���Ǹ���������ˮ������������Դ�����˷�ֹ�����ĸ�ʴ�⣬���н����Ļ������ã��������ɽ�����أ�Ѱ�ҽ����Ĵ���Ʒ�ȡ��ʴ�Ϊ�������ԡ�����������ˮ�������������պ����ã�
��2���������Ǻ�̼����ͬ���������Ͻ������ĺ�̼��Ϊ2��~4.3�����ֵĺ�̼��Ϊ0.03��~2�����������к�̼���ȸ��иߡ��ʴ�Ϊ�������ߣ�
��3���ɽ������˳�����֪��Al>Fe>Ag����AgNO3��Һ�м���һ������Al��Fe�Ļ�Ϸ�ĩ����������������Һ��Ӧ��������Ӧ���������������Һ��Ӧ���ɵõ���������dz��ɫ��Һ��˵������Һ�к�������������˵����������ȫ��Ӧ����������һ�������������ܺ�������һ��û��������Һ��һ���������������������������ܺ����������������Ϸ�����֪��
���������м���ϡ���ᣬ���������������������ݲ������ٴ���
����Һ��һ������Al(NO3)3��Fe(NO3)2�����ܺ�AgNO3��������Һ�м���ϡ���ᣬ�����г����������ڴ���
����Һ��һ������Al(NO3)3��Fe(NO3)2�����ܺ�AgNO3������ȷ��
��������һ��û������һ�����������ܴ���
�ʴ�Ϊ���ۡ�

 ������ҵ����ν�����������ϵ�д�
������ҵ����ν�����������ϵ�д�����Ŀ��ij��ѧ��ȤС��Ϊ�˲ⶨij��ͭ��ͭ��п�Ͻ���Ʒ��п������������ȡ10����Ʒ�����ձ��У���ȡ60��ϡ��������μ����ձ��У�����ַ�Ӧ��ʵ���������£�
��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� | ������ | |
����ϡ�����������g�� | 10 | 10 | 10 | 10 | 10 | 10 |
ʣ������������g�� | 9.35 | 8.7 | 8.05 | 7.4 | 6.75 | 6.75 |
��1����ͭ��Ʒ��п����������Ϊ���٣���д�����㲽�裬��ͬ��
��2������ϡ�������������Ϊ���٣�
����Ŀ��С���ڼ����������ĺ�����з���һ��˫���������ǩ��ͼ��ʾ���õ�ѧУ��ͬѧ�Ƕ�������õĹ�����ƷҲ�ܺ��棬��������ʦ��ָ���½���������̽����

��������⣩���ù���ijɷ���ʲô��
���������룩���ù����п��ܺ���Fe��Fe2O3��CaO��Ca(OH)2��CaCO3��
���������ϣ������Ȼ�����Һ�ڳ����·�����Ӧ�����Ȼ�������
��ʵ��̽����С������˷���һ��
ʵ����� | ʵ������ | ʵ����� |
ȡ������������Թ��У��μ�������______�� | ��������ʧ������ɫ���ݲ������õ�dz��ɫ��Һ�� | ������һ������____��һ������Fe2O3�� |
ͬѧ����ΪС�յķ���һ�����ܵó�һ������Fe2O3�Ľ��ۣ������ǣ�______��������ۺ��������ʵ�������������֤��
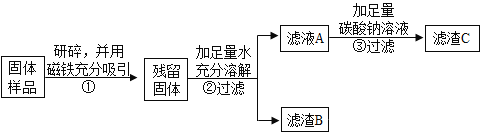
������й����ܽ�ʱ�ų��������ɴ˿����жϹ�����һ������________________�� д���ù����з�Ӧ�Ļ�ѧ����ʽ______________��
ͬѧ�Ƕ�����B�ٽ���̽����
ʵ����� | ʵ������ | ʵ����� |
ȡ����B���Թ��У���������ϡ���ᣬ���ɵ�����ͨ�����ʯ��ˮ | ������٣�__________��ʯ��ˮ����ǡ� | ������һ������CaCO3��Fe2O3�� |
��ʵ����ۣ� �þ��ù�����һ������Fe��Fe2O3��CaO��CaCO3��
����˼�� ʳƷ��װ���ڷ�˫������Ŀ����_________________________________��