��Ŀ����
ij��ѧС��ɹ��������ͼ��ʾʵ�飨װ�����������ã�����֤�˶�����̼����ɡ�
̽���������£�

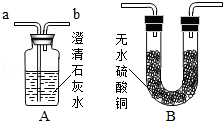
1. ����װ�и����״ľ̿���Թ�D������Ϊ50.7g��װ�м�ʯ�ҵ�װ��C����Ϊ112.3g������A��B��Dװ�ã�
2. �ӳ���©����������3%�Ĺ���������Һ������Cװ�ã���ȼ�ƾ��ƣ�
3.��D�з�����Ӧ��Ϩ��ƾ��ƣ���ȴ��
4. �����Թ�D��ʣ����������Ϊ50.1g��װ��C������Ϊ114.5g
��֪����ʯ�ҵijɷ��������ƺ��������ƣ�ľ̿�е����ʲ����뷴Ӧ��B��C����װҩƷ����������ȫ����������ʡ���ش��������⣺
װ��A��Ӧ�Ļ�ѧ����ʽΪ_____________________���÷�Ӧ����_________��Ӧ
�ƾ��Ƽ��ȵ�������_____________________
Ϊ��С���������ȴ��������Ҫע���������_______________________
����ʵ�����ݼ���μӷ�Ӧ������������Ϊ����ʽ�����㣩_____________���Ӷ������������̼��̼����Ԫ�ص������ȡ�
̽���������£�

1. ����װ�и����״ľ̿���Թ�D������Ϊ50.7g��װ�м�ʯ�ҵ�װ��C����Ϊ112.3g������A��B��Dװ�ã�
2. �ӳ���©����������3%�Ĺ���������Һ������Cװ�ã���ȼ�ƾ��ƣ�
3.��D�з�����Ӧ��Ϩ��ƾ��ƣ���ȴ��
4. �����Թ�D��ʣ����������Ϊ50.1g��װ��C������Ϊ114.5g
��֪����ʯ�ҵijɷ��������ƺ��������ƣ�ľ̿�е����ʲ����뷴Ӧ��B��C����װҩƷ����������ȫ����������ʡ���ش��������⣺
װ��A��Ӧ�Ļ�ѧ����ʽΪ_____________________���÷�Ӧ����_________��Ӧ
�ƾ��Ƽ��ȵ�������_____________________
Ϊ��С���������ȴ��������Ҫע���������_______________________
����ʵ�����ݼ���μӷ�Ӧ������������Ϊ����ʽ�����㣩_____________���Ӷ������������̼��̼����Ԫ�ص������ȡ�
��1��2H2O2 2H2O+O2�� �ֽ⣨2����ȼľ̿
2H2O+O2�� �ֽ⣨2����ȼľ̿
��3��������������4����114.5g-112.3g��-(50.7g-50.1g)=1.6g
 2H2O+O2�� �ֽ⣨2����ȼľ̿
2H2O+O2�� �ֽ⣨2����ȼľ̿��3��������������4����114.5g-112.3g��-(50.7g-50.1g)=1.6g
(1)װ��A�е�����Ϊ�����������������,������������������ӦΪ: 2H2O2 2H2O+O2������Ӧ�Ļ�������Ϊ�ֽⷴӦ��
2H2O+O2������Ӧ�Ļ�������Ϊ�ֽⷴӦ��
��2��D����������������ľ̿��Ӧ�����Ծƾ��Ƶ������ǵ�ȼľ̿��
��3���������ȴ�����л���ȴǰֹͣͨ�����������������¶Ƚ������D����ѹ���ͣ��Ӷ�����������C�С������к��е�ˮ������������̼�������ʯ�ҷ�Ӧ����ʹC�е������仯ֵƫ��һ��Ӱ��ʵ������
��4�����������غ㶨�ɣ����뷴Ӧ������������ӦΪ���ɵĶ�����̼������[C�����أ�Ϊ��114.5g-112.3g��]��ȥ���뷴Ӧ��ľ̿������[D�м��ٵ�������Ϊ(50.7g-50.1g)]��
����������Ϊʵ���ۺ���Ŀ����ϲⶨ������̼����ɣ���������������ȡ��ľ̿�������ķ�Ӧ�������غ㶨�ɵ�֪ʶ�㡣
 2H2O+O2������Ӧ�Ļ�������Ϊ�ֽⷴӦ��
2H2O+O2������Ӧ�Ļ�������Ϊ�ֽⷴӦ����2��D����������������ľ̿��Ӧ�����Ծƾ��Ƶ������ǵ�ȼľ̿��
��3���������ȴ�����л���ȴǰֹͣͨ�����������������¶Ƚ������D����ѹ���ͣ��Ӷ�����������C�С������к��е�ˮ������������̼�������ʯ�ҷ�Ӧ����ʹC�е������仯ֵƫ��һ��Ӱ��ʵ������
��4�����������غ㶨�ɣ����뷴Ӧ������������ӦΪ���ɵĶ�����̼������[C�����أ�Ϊ��114.5g-112.3g��]��ȥ���뷴Ӧ��ľ̿������[D�м��ٵ�������Ϊ(50.7g-50.1g)]��
����������Ϊʵ���ۺ���Ŀ����ϲⶨ������̼����ɣ���������������ȡ��ľ̿�������ķ�Ӧ�������غ㶨�ɵ�֪ʶ�㡣

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ
 �� ��
�� ��