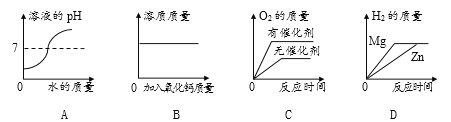
��Ŀ����
����Ŀ��������з���ʽ����ע���������Ӧ���͡�
(1)��������������������ԭ���ǣ��÷��мв��е�ˮ����ʯ�ҽӴ�����Ӧ���ų��������ȡ��÷�Ӧ�Ļ�ѧ����ʽ_______������_______��Ӧ��
(2)��������������Ļ�ѧ����ʽ_______������_______��Ӧ��
(3)þ������ͭ��Һ��Ӧ�Ļ�ѧ����ʽ_______������_______��Ӧ
(4)��˿��������ȼ�յĻ�ѧ����ʽ_______������_______��Ӧ��
(5)Fe2O3��ϡ���ᷴӦ�Ļ�ѧ����ʽ_______��
(6)��Ȼ����ȫȼ�յĻ�ѧ����ʽΪ_______��
���𰸡�CaO+H2O��Ca (OH)2 ���� 2KClO3![]() 2KCl+3O2�� �ֽ� Mg+CuSO4��MgSO4+Cu �û� 3Fe+2O2
2KCl+3O2�� �ֽ� Mg+CuSO4��MgSO4+Cu �û� 3Fe+2O2![]() Fe3O4 ���� Fe2O3+3H2SO4��Fe2 (SO4)3+3H2O CH4+2O2
Fe3O4 ���� Fe2O3+3H2SO4��Fe2 (SO4)3+3H2O CH4+2O2![]() CO2+2H2O
CO2+2H2O
��������
(1)�����ƺ�ˮ��Ӧ�����������ƣ���ѧ����ʽΪ��CaO+H2O��Ca(OH)2���÷�Ӧ���ڻ��Ϸ�Ӧ��
(2)������ڶ������̵Ĵ������¼��������Ȼ��غ���������ѧ����ʽΪ��2KClO3![]() 2KCl+3O2�����÷�Ӧ���ڷֽⷴӦ��
2KCl+3O2�����÷�Ӧ���ڷֽⷴӦ��
(3)þ������ͭ��Ӧ��������þ��ͭ����ѧ����ʽΪ��Mg+CuSO4��MgSO4+Cu���÷�Ӧ�����û���Ӧ��
(4)���������ڵ�ȼ��������������������������ѧ����ʽΪ��3Fe+2O2![]() Fe3O4���÷�Ӧ���ڻ��Ϸ�Ӧ��
Fe3O4���÷�Ӧ���ڻ��Ϸ�Ӧ��
(5)�����������ᷴӦ������������ˮ����ѧ����ʽΪ��Fe2O3+3H2SO4��Fe2 (SO4)3+3H2O��
(6)����������ڵ�ȼ�����������ɶ�����̼��ˮ����ѧ����ʽΪ��CH4+2O2![]() CO2+2H2O��
CO2+2H2O��
�ʴ�Ϊ��(1)CaO+H2O��Ca (OH)2�����ϣ�
(2)2KClO3![]() 2KCl+3O2�����ֽ⣻
2KCl+3O2�����ֽ⣻
(3)Mg+CuSO4��MgSO4+Cu���û���
(4)3Fe+2O2![]() Fe3O4�����ϣ�
Fe3O4�����ϣ�
(5)Fe2O3+3H2SO4��Fe2 (SO4)3+3H2O��
(6)CH4+2O2![]() CO2+2H2O��
CO2+2H2O��

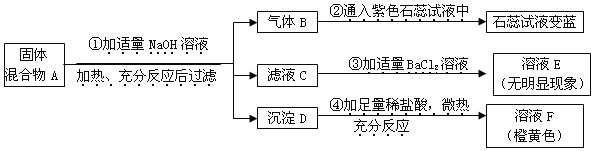
����Ŀ����һ�������£���һ���ܱ������ڷ���ij��Ӧ����÷�Ӧǰ������ʵ��������±���ʾ������˵���������(����)
�� �� | �� �� | ������̼ | ˮ���� | W |
��Ӧǰ����/g | 50 | 1 | 1 | 23 |
��Ӧ������/g | 2 | 45 | 28 | x |
A. ���������غ㶨�ɣ�x��ֵӦΪ0 B. ��Ӧ����������Ƕ�����̼��ˮ
C. ����W��̼���⡢������Ԫ�� D. ����Wֻ��̼��������Ԫ��