��Ŀ����
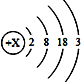
����Ŀ�����䰮�������ܾ���Ʒ���������������Ӧ��Ʒ˵������������ʳ������(����������C10H15N)���������ú�ǿ���������ƻ��˵������������ܡ������й�˵������ȷ����
A. �����������л��� B. ������������Է�������Ϊ149
C. ÿ�������������Ӻ���26��ԭ�� D. ����������C��H��N��������Ϊ10:15:1
���𰸡�D
��������A. �����������л����ȷ��
B. ������������Է�������Ϊ12![]() =149������ȷ��
=149������ȷ��
C��ÿ�������������Ӻ���10��̼ԭ�ӡ�15����ԭ�Ӻ�1����ԭ�ӣ�����26��ԭ�ӣ���ȷ��
D������������C��H��N��ԭ�Ӹ�����Ϊ10:15:1���������Ȳ�������10:15:1���ʴ���ѡD��

��ϰ��ϵ�д�
�����Ŀ