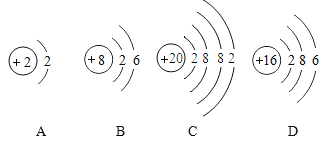
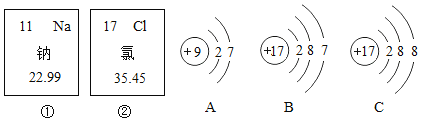
��Ŀ����
����Ŀ��Ϊ�ⶨһƿ��ǩ�ѱ����ָ�ʴ�Ĺ���������Һ������������������������ռ��Һ�ĺ�������С����չ������̽����
���������������װ�������ԡ�
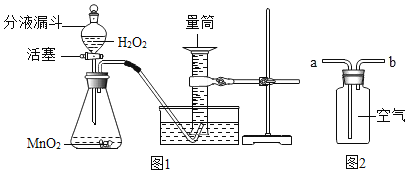
��ȡ�����Ķ������̷�ĩ������ƿ�У���������ƽ��ȡһ�������Ĺ������⣨H2O2����Һ�������Һ©���С�
����ͼ1��ʾ��װ��ʵ��װ�á�
������Һ©����������ȡ������������������������ݸ��¶�ʱ�������ܶȼ����������������Ͳ�㹻���ܹ��ռ���������������
�����������������������ù�������������֮�������������ϵ������������������ù���������������Գ����Ĺ���������Һ�����ó�����������Һ��������������
����������ƿ�еĶ������̣�ϴ�������Ż�ԭ����
�ش��������⣺
��1���÷�Ӧ�ı���ʽΪ__��
��2����ʵ���У������Ƿ�Ӧ�¶ȶ�ʵ���Ӱ�죬��ȴ��������ռ����������__�����������������������������С������������������
��3����ȡ���������ʱ��������Ӷ�������������__����������������С����������������ʵ��ֵ��
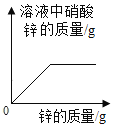
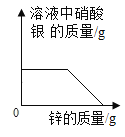
��4��������ͼ2�ռ�����������Ӧ�ô�__����a������b����ͨ�롣
��5�����������ڸ�ʵ���е�������__��
���𰸡���������![]() ˮ+������H2O2
ˮ+������H2O2![]() H2O+O2 ���� ���� a ����
H2O+O2 ���� ���� a ����
��������
��1�����������ڶ������̴������·ֽ�����ˮ����������Ӧ�����ֱ���ʽ��ѧ���ű���ʽΪ����������![]() ˮ+������H2O2
ˮ+������H2O2![]() H2O+O2��
H2O+O2��
��2����ʵ���У������Ƿ�Ӧ�¶ȶ�ʵ���Ӱ�죬��ȴ��������ռ�������������ڲ������������������Ϊ����������Һ������ƿ��ռ��һ�������
��3����ȡ���������ʱ��������Ӷ�����������������ʵ��ֵ��
��4��������ͼ2�ռ����������������ܶȱȿ���������Ӧ�ô�aͨ�룻
��5�����������ڸ�ʵ���е������Ǵ�����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ϊ̽��CO2��NaOH��Һ�����ķ�Ӧ��ij��ȤС�鳢���ò�ͬ�ķ�ʽ����ʵ�顣
���������ϣ���20��Cʱ������������ˮ�е��ܽ�ȼ��±���
���� | Na2CO3 | NaHCO3 | Ca��OH��2 | Ba��OH��2 |
�ܽ�� | 21.5 | 9.6 | 0.165 | 3.89 |
��ʵ�������£�Na2CO3��Һ��NaHCO3��Һ��pH�ֱ�ԼΪ11.0��8.5��
��ʵ��̽����
��1��ʵ��һ��С��ȡһ����CO2�Ŀ�Ȫˮƿ������һ������ˮ������š��ƿ�ǣ�������ƿ�ӱ��С����ȡһ��ͬ��С�ij���CO2�Ŀ�Ȫˮƿ�������м�����ˮ�������NaOH��Һ������š��ƿ�ǣ����õ���ҺA����ʱ�۲쵽��������__________��������ֻ��Ȫˮƿ��ʵ���Ŀ����_______________��
��2��ʵ�����Ϊ����CO2��NaOH��Һ��Ӧ�IJ��С��ȡʵ��һ������ҺA�����������еμ�������ϡ���ᣬ�۲쵽____________����֤����Ӧ�����ˡ�
��3��ʵ������Ϊ����CO2��NaOH��Һ��Ӧ�IJ��С��ȡʵ��һ������ҺA�����������еμ�BaCl2��Һ���а�ɫ�����������÷�Ӧ�Ļ�ѧ����ʽΪ_________��ʵ���в��˽�BaCl2��Һ����CaCl2��Һ��ԭ����__________��
��4��ʵ���ģ�С��ȡʵ��һ������ҺA�����������м��������BaCl2��Һ�������ã�ȡ�ϲ���Һ������1�η�̪��Һ��������Һ��___ɫ��֤����ҺA����NaOHʣ�ࡣʵ���У�С��û��ֱ����������ҺA�е����̪��Һ��������_________________��
��5��ʵ���ģ���ȤС��CO2����ͨ��һ��Ũ��һ������NaOH��Һ�У������ֻ�ʵ�鼼���ⶨ��Ӧ��������Һ��pH���¶ȱ仯�������ͼ1��ͼ2��ʾ��
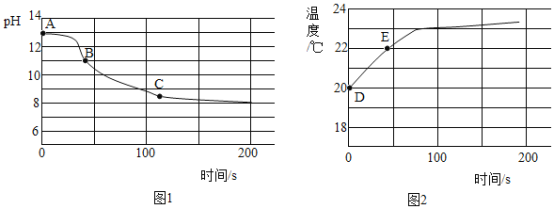
ͼ1�У�BC�η�����Ӧ�Ļ�ѧ����ʽΪ__________________��
ͼ2�У�DE���¶ȱ仯��ԭ����___________________��
����˼����5��ʵ���CO2���١�NaOH���٣�Na2CO3���ɵ����ʵı仯���Լ�____________�仯���ӽǶ�ά��̽��CO2��NaOH�����˷�Ӧ�������������ԵĻ�ѧ��Ӧ������ͨ���ִ������ֶν������ݲⶨ��ʵ�ַ�Ӧ���̵������ӻ�����