��Ŀ����
ij����С���һ�����ŷŵķ�ˮ�к���H2SO4��CuSO4��Ϊ�˲ⶨ��ˮ��CuSO4��������������С��ȡ��100g��ˮ����μ���10%��NaOH��Һ���������������Cu��OH��2��������������NaOH��Һ������ϵ����ͼ��ʾ����1����H2SO4��Ӧ��NaOH��Һ����Ϊ______g��100g��ˮ��H2SO4������Ϊ______g��
��2�������ˮ��CuSO4������������
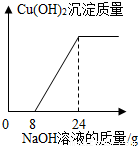
���𰸡����������ݡ�����Cu��OH��2��������������NaOH��Һ������ϵ��ͼ��������Ӧ���е������Ȼ��ֱ���ݷ�Ӧ�Ļ�ѧ����ʽ���м��㣮
����⣺��1���ɹ�ϵͼ��֪����8gNaOH��Һ���ˮ�е����ᷢ����Ӧ��
���ˮ����������Ϊx
2NaOH+H2SO4=Na2SO4+2H2O
80 98
8g×10% x
80��98=��8g×10%����x
��֮�� x=0.98g
�ʴ�Ϊ��8��0.98��
��2���ɹ�ϵͼ��֪�����ˮ������ͭ��Ӧ��NaOH��Һ������=24g-8g=16g������NaOH������=16g×10%=1.6g
���ˮ������ͭ������Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 80
y 1.6g
160��80=y��1.6g
��֮�� y=3.2g
��ˮ��CuSO4����������= ×100%=3.2%
×100%=3.2%
�𣺷�ˮ��CuSO4����������Ϊ3.2%��
��������Ӧ�Ĺ�ϵͼ�У�����8gNaOH��Һʱ�ſ�ʼ���ֳ������������֮ǰ�������NaOH��Һ���ˮ�е����ᷴӦ����ˮ�����ᱻ��Ӧ�꣬�������NaOH��Һ��ʼ���ˮ�е�����ͭ��Ӧ��ֱ������24gNaOH��Һ��Ӧȫ����ɣ�
����⣺��1���ɹ�ϵͼ��֪����8gNaOH��Һ���ˮ�е����ᷢ����Ӧ��
���ˮ����������Ϊx
2NaOH+H2SO4=Na2SO4+2H2O
80 98
8g×10% x
80��98=��8g×10%����x
��֮�� x=0.98g
�ʴ�Ϊ��8��0.98��
��2���ɹ�ϵͼ��֪�����ˮ������ͭ��Ӧ��NaOH��Һ������=24g-8g=16g������NaOH������=16g×10%=1.6g
���ˮ������ͭ������Ϊy
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 80
y 1.6g
160��80=y��1.6g
��֮�� y=3.2g
��ˮ��CuSO4����������=
 ×100%=3.2%
×100%=3.2%�𣺷�ˮ��CuSO4����������Ϊ3.2%��
��������Ӧ�Ĺ�ϵͼ�У�����8gNaOH��Һʱ�ſ�ʼ���ֳ������������֮ǰ�������NaOH��Һ���ˮ�е����ᷴӦ����ˮ�����ᱻ��Ӧ�꣬�������NaOH��Һ��ʼ���ˮ�е�����ͭ��Ӧ��ֱ������24gNaOH��Һ��Ӧȫ����ɣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
ij����С���һ�����������ŷ����Է�Һ�к���CuCl2����С��ȡ��100g��Һ���ձ��У���28gһ����������������NaOH��Һ���Ĵμ��룬������ɵij�������������NaOH��Һ��������ϵ�������±���
�Լ��㣺������������һλС����
��1��100g��Һ�е�CuCl2��ȫ��Ӧ�����ij�������Ϊ g��
��2����Һ��CuCl2�������Ƕ��ٿˣ�
��3������NaOH��Һ����������������
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ��������������Һ������ | 4g | 12g | 20g | 28g |
| ���ɳ��������� | 0 | 0.98g | 1.96g | 1.96g |
��1��100g��Һ�е�CuCl2��ȫ��Ӧ�����ij�������Ϊ
��2����Һ��CuCl2�������Ƕ��ٿˣ�
��3������NaOH��Һ����������������
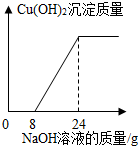 ij����С���һ�����ŷŵķ�ˮ�к���H2SO4��CuSO4��Ϊ�˲ⶨ��ˮ��CuSO4��������������С��ȡ��100g��ˮ����μ���10%��NaOH��Һ���������������Cu��OH��2��������������NaOH��Һ������ϵ����ͼ��ʾ��
ij����С���һ�����ŷŵķ�ˮ�к���H2SO4��CuSO4��Ϊ�˲ⶨ��ˮ��CuSO4��������������С��ȡ��100g��ˮ����μ���10%��NaOH��Һ���������������Cu��OH��2��������������NaOH��Һ������ϵ����ͼ��ʾ��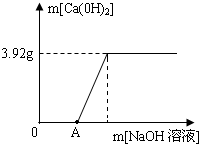 ij����С���һ�������ˮ���ŷŵ����Է�Һ�к���CuSO4��
ij����С���һ�������ˮ���ŷŵ����Է�Һ�к���CuSO4��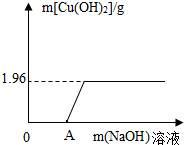 ij����С���һ����������ŷŵ����Է�Һ�к���CuCl2��Ϊ�˲ⶨ��Һ��CuCl2��������������С��ȡ��100g��Һ����ε���NaOH��Һ���������������Cu��OH��2����������NaOH��Һ��������ϵ��ͼ��
ij����С���һ����������ŷŵ����Է�Һ�к���CuCl2��Ϊ�˲ⶨ��Һ��CuCl2��������������С��ȡ��100g��Һ����ε���NaOH��Һ���������������Cu��OH��2����������NaOH��Һ��������ϵ��ͼ�� ij����С���һ����������ŷŵ����Է�Һ�к���CuCl2��Ϊ�˲ⶨ��Һ��CuCl2��������������С��ȡ��100g��Һ����ε���NaOH��Һ���������������Cu��OH��2����������NaOH��Һ��������ϵ��ͼ��
ij����С���һ����������ŷŵ����Է�Һ�к���CuCl2��Ϊ�˲ⶨ��Һ��CuCl2��������������С��ȡ��100g��Һ����ε���NaOH��Һ���������������Cu��OH��2����������NaOH��Һ��������ϵ��ͼ��