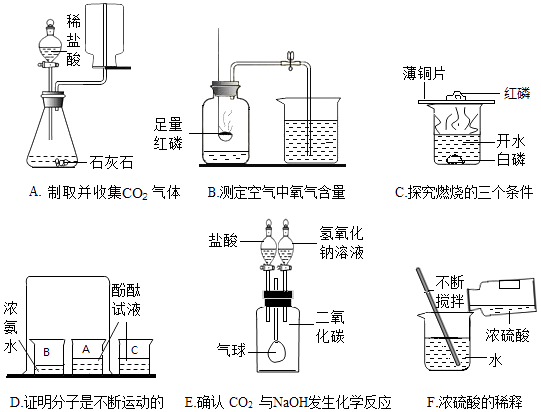
��Ŀ����
����Ŀ����������������CO2�ĺ���������������������ЧӦ��ǿ��ȫ�������ů��
��1����Ȼ�������մ�����CO2������Ҫ������ ��
��2����ѧ���о����֣���ˮ�����մ���CO2 �� ���CO2�ŷ����������ӣ��������ữ������д�������ữԭ�����û�ѧ��Ӧ����ʽ��ʾ����
��3����ѧ��Ŀǰ�����о����������й�����CO2��H2 �� �ڴ��������������£�ת����Һ̬�״���CH3OH����ˮ����Ӧ�Ļ�ѧ����ʽΪ�� ��
���𰸡�
��1���������
��2��CO2+H2O�TH2CO3
��3��CO2+3H2 ![]() CH3OH+H2O
CH3OH+H2O
���������⣺��1������ֲ�������õ�ԭ��֮һ�Ƕ�����̼��������Ȼ�������մ�����CO2������Ҫ�����ǹ�����ã���2����ѧ���о����֣���ˮ�����մ���CO2 �� ���ڶ�����̼��ˮ��Ӧ����̼�ᣮ���������̼�ŷ����������ӣ��������ữ������Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O�TH2CO3 �� ��3���������֪�������й�����CO2��H2 �� �ڴ��������������£�ת����Һ̬�״���CH3OH����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+3H2 ![]() CH3OH+H2O�� �ʴ�Ϊ����1��������ã���2��CO2+H2O�TH2CO3����3��CO2+3H2
CH3OH+H2O�� �ʴ�Ϊ����1��������ã���2��CO2+H2O�TH2CO3����3��CO2+3H2 ![]() CH3OH+H2O��
CH3OH+H2O��
�����㾫�����������⣬������Ҫ�˽������̼�Ļ�ѧ����(��ѧ���ʣ�һ������²���ȼ��,Ҳ��֧��ȼ�գ����ܹ�����������ˮ��Ӧ����̼���ʹ�����ʯ��ˮ����ǣ������ȵ�̼��Ӧ)����Ҫ������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ(ע�⣺a����ƽ b������ c������)�����֪ʶ���Ǵ���Ĺؼ���

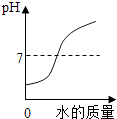
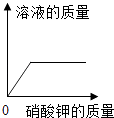
����Ŀ�������ĸ�ͼ���У�����ȷ��ʾ�Է�Ӧ�仯��ϵ���ǣ� ��
|
|
|
|
A����һ����ϡ�����м�ˮϡ�� | B��һ���¶��£������������Һ�м�������� | C������һ�����ĸ�����ع��� | D����������Ȼ�þ�Ļ����Һ�м�����������Һ |
A.A
B.B
C.C
D.D