��Ŀ����
С����ⶨCu-Zn�Ͻ��Cu-Ag�Ͻ���ͭ������������ʵ����ֻ�ṩ��һƿϡ�������ص���������1���������е�����������Ϊֻ�ܲ��______�Ͻ���ͭ������������
��2��Ϊ�˲ⶨ�úϽ����ɣ�С����ȡ10g�úϽ��ĩ���ڷ�ĩ���������μ���ϡ���ᷴӦ��ÿ��һ�����ᣬС����¼���������������ʵ���������£�
| ��һ�� | �ڶ��� | ������ | |
| ������������������ml�� | 10 | 10 | 10 |
| ����������������g�� | 0.08 | 0.08 | 0.04 |
��4������úϽ���ͭ������������
���𰸡��������ڽ������˳���У���ǰ�Ľ��������ᷴӦ����������������ⶨ�Ͻ���ͭ���������������е���һ�ֽ���������ǰ�����ݱ����ṩ�����ݣ��Ϳ�֪�������������������������ɵ�������������úϽ���п���������������ͭ������������
����⣺
��1���ڽ������˳���У���ǰ�Ľ��������ᷴӦ����������������ⶨ�Ͻ���ͭ���������������е���һ�ֽ���������ǰ�����Ա����Ϊ��Cu-Zn��
��3�����ݱ����ṩ�����ݣ�10g�����������0.08g������������ֻ����0.04g��˵������ȫ���μ��˷�Ӧ��������������������Ϊ��0.08g+0.08g+0.04g=0.2g�����Ա����Ϊ��0.2g��
��4���⣺��Cu-Zn�Ͻ���п������Ϊx��
Zn+2HCl�TZnCl2+H2��
65 2
x 0.2g
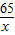 =
=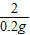 X=6.5g
X=6.5g
Cu-Zn�Ͻ���ͭ����������Ϊ��
 ×100%=35%
×100%=35%
��Cu-Zn�Ͻ���ͭ����������Ϊ35%��
���������⿼���˺Ͻ���ij�ֽ��������IJⶨ����ɴ��⣬�������ݽ������˳��������Լ��йػ�ѧ����ʽ�ļ�����У�
����⣺
��1���ڽ������˳���У���ǰ�Ľ��������ᷴӦ����������������ⶨ�Ͻ���ͭ���������������е���һ�ֽ���������ǰ�����Ա����Ϊ��Cu-Zn��
��3�����ݱ����ṩ�����ݣ�10g�����������0.08g������������ֻ����0.04g��˵������ȫ���μ��˷�Ӧ��������������������Ϊ��0.08g+0.08g+0.04g=0.2g�����Ա����Ϊ��0.2g��
��4���⣺��Cu-Zn�Ͻ���п������Ϊx��
Zn+2HCl�TZnCl2+H2��
65 2
x 0.2g
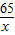 =
=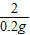 X=6.5g
X=6.5gCu-Zn�Ͻ���ͭ����������Ϊ��
 ×100%=35%
×100%=35%��Cu-Zn�Ͻ���ͭ����������Ϊ35%��
���������⿼���˺Ͻ���ij�ֽ��������IJⶨ����ɴ��⣬�������ݽ������˳��������Լ��йػ�ѧ����ʽ�ļ�����У�

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ
С����ⶨCu-Zn�Ͻ��Cu-Ag�Ͻ���ͭ������������ʵ����ֻ�ṩ��һƿϡ�������ص�������
��1���������е�����������Ϊֻ�ܲ�� �Ͻ���ͭ������������
��2��Ϊ�˲ⶨ�úϽ����ɣ�С����ȡ10g�úϽ��ĩ���ڷ�ĩ���������μ���ϡ���ᷴӦ��ÿ��һ�����ᣬС����¼���������������ʵ���������£�
��3�����ϱ����ݷ�����С����10g�Ͻ��ĩ�ܹ��ռ��� g������
��4������úϽ���ͭ������������
��1���������е�����������Ϊֻ�ܲ��
��2��Ϊ�˲ⶨ�úϽ����ɣ�С����ȡ10g�úϽ��ĩ���ڷ�ĩ���������μ���ϡ���ᷴӦ��ÿ��һ�����ᣬС����¼���������������ʵ���������£�
| ��һ�� | �ڶ��� | ������ | |
| ������������������ml�� | 10 | 10 | 10 |
| ����������������g�� | 0.08 | 0.08 | 0.04 |
��4������úϽ���ͭ������������