��Ŀ����
����Ŀ������ͼһ��ͼ����ͼ��,�ش���������:
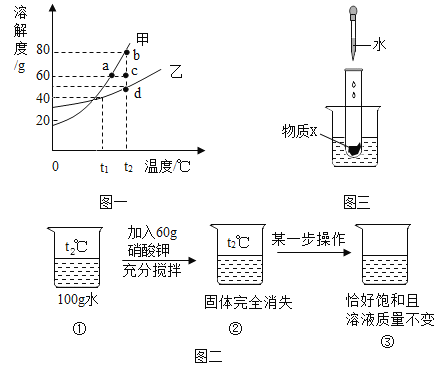
��1����ͼ���Ʋ��������ͼһ�е�_____���ʡ�
��2��t2��Cʱ,��1981.7 g�����ʵı�����Һ������10 gˮ,�ٽ��µ�t2 ��C ,�����������ʵ�����Ϊ__��
��3����t2��Cʱ�����������ʵı�����Һ������t1��Cʱ��������Һ���������������Ĵ�С��ϵ�Ǽ�__�ң�����>����<������=������
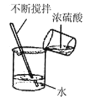
��4��ͼ����ijһ���������ɰ� ��ͼ����ʾ,��ˮ���뵽ʢ����������X��С�Թ��е���ʵ�֣�������X������____��ѡ����ţ�
����ʯ�� ��Ũ���� ������� ����������
���𰸡��� 8 g > ��
��������
��1����ͼ�����Կ�����t2��ʱ������ص��ܽ�ȴ���60g�������Ʋ��������ͼһ�еļ����ʣ�
��2��t2��ʱ������ص��ܽ����80g��10g��ˮ��8g������γɱ�����Һ�����Դ�1981.7g�����ʵı�����Һ������10gˮ���ٽ��µ�t2�棬�����������ʵ�����Ϊ8g��
��3����t2��ʱ�����������ʵı�����Һ������t1��ʱ���ס������ʵ��ܽ�ȶ���С����ȻΪ������Һ�������ʵ��ܽ�ȴ��������ʵ��ܽ�ȣ�������Һ���������������Ĵ�С��ϵ�Ǽף��ң�
��4��ͼ�����еĹ�����ȫ��ʧ����Һ���ܸպñ��ͣ�Ҳ���ܲ����ͣ���ijһ������������Һǡ�ñ��ͣ�����Һ�������䣬Ӧ�ý����е���Һ���£��������Կɰ���ͼ����ʾ����ˮ���뵽ʢ����������X��С�Թ��е���ʵ�֣�������X����������李�

����Ŀ��25��ʱ��̽��ij�������ʵ��ܽ��ԣ�ʵ���¼���±�������ʵ�������ȷ����
��� | �� | �� | �� | �� |
ˮ������/g | 50 | 50 | 50 | 50 |
�����������/g | 5 | 10 | 15 | 20 |
���� | ������ȫ�ܽ� | ������ȫ�ܽ� | ʣ���������� | ʣ��϶���� |
A. ʵ���������Һ��������Ϊ10%
B. ʵ��˵��25��ʱ�����ʵ��ܽ����20g
C. ʵ��ۢ�������Һ����������ͬ
D. ʵ���������Һ�к�����20g
����Ŀ��þ���ڿ����о��ñ�����ڣ�ijС��ͬѧ��Ʋ�����ʵ��̽��þ����ڵ�������
���������ϡ���ʯ�ҿ����ն�����̼��ˮ��
����������衿�����£�þ����ڿ�����O2��CO2��ˮ�����й�
������ʵ�顿ͨ��������þ���Ӵ������ʣ�������ͼװ��(þ������Ϊ3cm���Թ��ݻ�Ϊ20mL)���ֱ��������5��ʵ�飬�������۲�20�졣
��� | 1 | 2 | 3 | 4 | 5 |
ʵ�� |
|
|
|
|
|
���� | �����Ա仯 | �����Ա仯 | �����Ա仯 | �����Ա仯 | 20���þ����� |
����������ۡ�
(1)ʵ��1��Ŀ����________��
(2)ʵ��2�У�NaOH��Һ���տ����ж�����̼�Ļ�ѧ����ʽ��________��
(3)�ó���þ�����һ����CO2�й������ۣ����ݵ�����ʵ����________��(����)
(4)������ʵ�����֪��þ����ڵ�������________��
����˼�����ۡ�
(5)�ڲ��������ʱ��ͬѧ����Ϊþ�������N2�أ���������________��