��Ŀ����
��ͼ������ͬѧ��ʵ����������ع�������һ�������������������Һ���ܵ�����������

�ش��������⣺
��1��E�������� ������ʱ�����������г�B��C��E�⣬������ѡ�õ��� ������ĸ��ţ�
��2����ʵ���У�C���������� ����ʹE��ȡˮʱ�������ָ��Ӱ�Һ����ʹ����������������Һ��Ũ�Ƚ� ��ƫС��ƫ����Ӱ�죩��
��3�����¶Ȳ���������£�������ʹ��������ʵ�����������Ϊԭ��2���ķ���������е���
A����10g�ӽ������������Һ������5gˮ
B����10g10%���������Һ�м���5g 40%���������Һ
C����10g����ز�������Һ�м���10g����ع��壮

�ش��������⣺
��1��E��������
��2����ʵ���У�C����������
��3�����¶Ȳ���������£�������ʹ��������ʵ�����������Ϊԭ��2���ķ���������е���
A����10g�ӽ������������Һ������5gˮ
B����10g10%���������Һ�м���5g 40%���������Һ
C����10g����ز�������Һ�м���10g����ع��壮
���㣺һ������������������Һ������,��������-��Ͳ,���ʵ���������
ר�⣺��Һ����Һ���ܽ��
��������1��ʵ��������һ������������ʳ����Һ�IJ�������ֱ��ǣ����㡢�������ܽ⣬���ݸ���������Ҫʹ�õ��������ж����������Ƿ���Ҫ��
��2��C������������ƽ�����ڳ�ȡ�����Ȼ��ƣ�����Ͳ��ȡˮʱ��С�����Ӱ�Һ�����ʹ�������������ʵ��Һ������ݴ��ж϶���������������Ӱ�죮
��3��A����10g����ر�����Һ������5gˮ������ˮ������������Һ��Ϊ������Һ��һ���¶��±�����Һ��������������=
��100%��
B����10g10%���������Һ�У�����5g40%���������Һ����Ϻ���Һ������Ϊ����Һ�����͡���������Ϊ����Һ�����������ͣ�����������������ʽ�����Ϻ���Һ������������
C����10g����ز�������Һ�м���10g����ع��壬�ܷ�ȫ���ܽ����жϣ���ԭ��Һ������������������ȷ����ȷ�е�֪������Һ����������������
��2��C������������ƽ�����ڳ�ȡ�����Ȼ��ƣ�����Ͳ��ȡˮʱ��С�����Ӱ�Һ�����ʹ�������������ʵ��Һ������ݴ��ж϶���������������Ӱ�죮
��3��A����10g����ر�����Һ������5gˮ������ˮ������������Һ��Ϊ������Һ��һ���¶��±�����Һ��������������=
| �ܽ�� |
| 100g+�ܽ�� |
B����10g10%���������Һ�У�����5g40%���������Һ����Ϻ���Һ������Ϊ����Һ�����͡���������Ϊ����Һ�����������ͣ�����������������ʽ�����Ϻ���Һ������������
C����10g����ز�������Һ�м���10g����ع��壬�ܷ�ȫ���ܽ����жϣ���ԭ��Һ������������������ȷ����ȷ�е�֪������Һ����������������
����⣺��1��E��������������Ͳ��������ƽ���ڳ�ȡ�����Ȼ��ơ���Ͳ�뽺ͷ�ι�����ȷ��ȡˮ���ձ���������ܽ�����������������ܽ�ʱ�Ľ��裬������ʱ������������г�B��C��E�⣬������ѡ�õ���F��H��
��2��C������������ƽ���ڸ�ʵ���У������ڳ�ȡ�Ȼ��ƣ�����Ͳ��ȡˮʱ��С�����Ӱ�Һ�����ʹ�������������ʵ��Һ����������ʵ����ȡ��ˮ�����ƫС����ʹ������������ƫ��
A���¶Ȳ��䣬����ص��ܽ�Ȳ��䣬������Һ�����������������䣻��A�����У�
B����10g10%���������Һ�У�����5g40%���������Һ����Ϻ���Һ��������������=
��100%=20%��Ϊԭ��Һ10%��2������C��ȷ��
C����ԭ��Һ����������������֪���Ҽ���10 gͬ�����ʺ��ܷ���ȫ�ܽⲻ��ȷ������������Һ������������һ������Ҫ��D�����У�
��ѡB��
�ʴ�Ϊ����1����Ͳ��F��H����2����ȡ�Ȼ��ƣ�ƫ��3��B��
��2��C������������ƽ���ڸ�ʵ���У������ڳ�ȡ�Ȼ��ƣ�����Ͳ��ȡˮʱ��С�����Ӱ�Һ�����ʹ�������������ʵ��Һ����������ʵ����ȡ��ˮ�����ƫС����ʹ������������ƫ��
A���¶Ȳ��䣬����ص��ܽ�Ȳ��䣬������Һ�����������������䣻��A�����У�
B����10g10%���������Һ�У�����5g40%���������Һ����Ϻ���Һ��������������=
| 10g��10%+5g��40% |
| 10g+5g |
C����ԭ��Һ����������������֪���Ҽ���10 gͬ�����ʺ��ܷ���ȫ�ܽⲻ��ȷ������������Һ������������һ������Ҫ��D�����У�
��ѡB��
�ʴ�Ϊ����1����Ͳ��F��H����2����ȡ�Ȼ��ƣ�ƫ��3��B��
�����������ѶȲ�����ȷ����һ������������������Һʵ�鲽�衢����������������������������������أ���������������ƫ����ܼ�����ƫС������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ



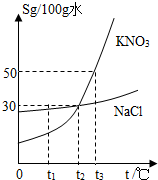 ��ͼ��KNO3��NaCl���ֹ������ʵ��ܽ�����ߣ�
��ͼ��KNO3��NaCl���ֹ������ʵ��ܽ�����ߣ�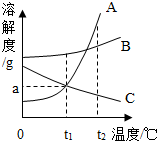 ��ͼΪA��B��C���ֹ������ʵ��ܽ�����ߣ���ͼ�ش��������⣮
��ͼΪA��B��C���ֹ������ʵ��ܽ�����ߣ���ͼ�ش��������⣮