��Ŀ����
�ᡢ��ζ�����Ҫ�Ļ������ش������й����⡣
��1�����в����ڸ��ֽⷴӦ������������ ������ĸ����
| A����ˮ���� | B������������ |
| C��������� | D�������������� |

��3������һ����������������������Һ���йز�������ͼ��ʾ��
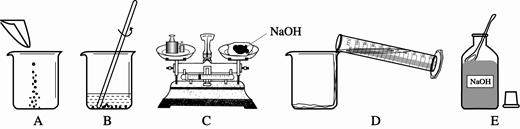
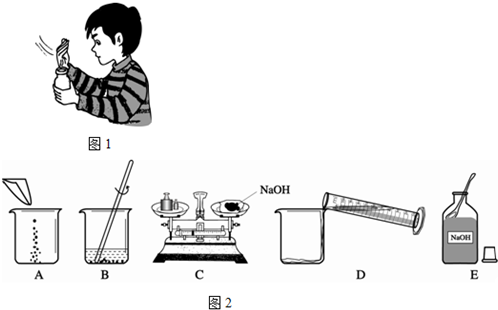
����ʵ�����ȷ����˳��Ϊ ��������ĸ���ű�ʾ��������B�е����������� ��C�����г��ֵĴ����� ����C�����������Ϊ15g������Ķ���Ϊ3.5g�����������ƺ�ֽƬ��ʵ��������Ϊ g��
��4����pH��ֽ�ⶨ��������Һ�����ȣ�Ӧ����β�����
��5����ҵ�ϳ��������������кͷ�ˮ�е����ᣬ��ȡ��Һ50g����ε���10%������������Һ�кͣ�����ҺpHΪ7ʱ����ȥ����������Һ8g�������ˮ���Ȼ��������������
��1��C��
��2��������ƿ��������ȶ����ü���������Ʈ���ǿף���Ҫ�ѱǿ״յ�ƿ��ȥ����ζ����
��3��ECADB�����������ձ�������ͱ��������ʷŵߵ��ˣ�11.5g��
��4���ڰ״ɰ����Ƭ�Ϸ�һСƬpH��ֽ��������Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�����Һ�����ȡ�
��5��1.46%
���������������1�����ֽⷴӦ��ָ�����ֻ����ﷴӦ�������������ֻ�����ķ�Ӧ�����ֽⷴӦ����������������������������ˮ�����ѡ��Cѡ�
��2����Ũ������ζʱ��Ҫע�ⲻ�ܰѱǿ״յ�ƿ��ȥ����ζ����Ӧ������ƿ��������ȶ����ü���������Ʈ���ǿ��ɣ�
��3��������Һ�IJ����ǣ��������ܽ⣬�����ȷ�IJ���˳��Ӧ����ECADB��B�е���������Ϊ���������ձ���C����������ͱ��������ʷŵߵ��ˣ���C�����������Ϊ15g������Ķ���Ϊ3.5g�����������ƺ�ֽƬ��ʵ��������Ϊ15g-3.5g="11.5g" ��
��4����pH��ֽ�ⶨ��������Һ��pHֵ�ķ����ǣ��ڰ״ɰ����Ƭ�Ϸ�һСƬpH��ֽ��������Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�����Һ��pHֵ��
��5���⣺���Һ���Ȼ��������Ϊx��
NaOH �� HCl��NaCl��H2O
40 36.5
8g��10% x 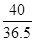 ��
��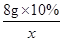
x��0.73
�Ȼ������������Ϊ��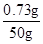 ��100%��1.46%
��100%��1.46%
���㣺���ֽⷴӦ�Ķ��壻����ʵ�������������Һ�IJ��裻��Һ�����ȵIJⶨ����ѧ����ʽ�ļ���
���������⿼���֪ʶ��϶࣬���ѶȲ������Ĺؼ������˽⸴�ֽⷴӦ�Ķ��壬���ջ���ʵ���������������Һ�IJ��衢��Һ�����ȵIJⶨ���Լ����ջ�ѧ����ʽ�ļ��㡣ѧ��Ӧע�⻯ѧ������Ͻ����淶��ע��������Ҫ��ϸ��

��1��������������ֻ�Ϳ����е�ˮ�����йص���
A�������ռ�Ҫ�ܷ���
B����ʯ�ҳ����ڿ����з��û����
C��Ũ����¶���ڿ����л����ء���ϡ
D������Ʒ���ڳ�ʪ�����л�����
��2��������һЩʳ��Ľ���pH������θ�������˱Ƚ����˵�ʳ����
| ѡ�� | A | B | C | D |
| ���� | ���� | �ݲ� | ���� | ������ |
| pH | 2.9��3.5 | 3.0��4.0 | 3.5��4.5 | 6.8-8.0 |
A��ϡ��Ũ����ʱ��Ϊʲô���ɽ�ˮ����Ũ��������Ҫ������ԭ��
B������������ᶼ��ʹ��ɫʯ����Һ��죬Ϊʲô��
C��д����ϡ�����ˮ������Ҫ�ɷ���̼��ƣ��Ļ�ѧ����ʽ��
��4����֧�Թ��зֱ�װ��������ɫ���壬���Ƿֱ����Ȼ��ơ��������ơ�̼����е�һ�֣�ֻҪ����������
��5��ʵ���������ʱijͬѧ������������ʵ�飺������ͭ��ϡ���ᷴӦ�����ռ���Һ���̪��Һ��Ӧ��ʵ�������ͬѧ���ַ�Һ��������ɫ����������д������ɫ�������ɵķ�Ӧ�Ļ�ѧ����ʽ��
��6����ͼ��Ũ�����Լ�ƿ�ϱ�ǩ�IJ������ݣ���ش�
A����Ҫ����9%��ϡ����120g����Ҫ����Ũ����
B��Ũ����ʹ��һ��ʱ�����������������С��ԭ����ʲô��
C��ij�������������ڿ����г��ڷ���һ��ʱ��ֱ��ʣ����ù���ȡ10g������������Ϊ9%�����������ٲ�������Ϊֹ����������������Ϊ2.2g���Լ���δ���ʵ��������Ƶ�����Ϊ���ٿˣ�
������ᡢ��ζ�����Ҫ����������밴Ҫ��ش�����һϵ����ص����⡣
��1��������������ֻ�Ϳ����е�ˮ�����йص��� �� ��
A�������ռ�Ҫ�ܷ���
B����ʯ�ҳ����ڿ����з��û����
C��Ũ����¶���ڿ����л����ء���ϡ
D������Ʒ���ڳ�ʪ�����л�����
��2��������һЩʳ��Ľ���pH������θ�������˱Ƚ����˵�ʳ���ǣ� ��
| ѡ�� | A | B | C | D |
| ���� | ���� | �ݲ� | ���� | ������ |
| pH | 2��9��3��5 | 3��0��4��0 | 3��5��4��5 | 6��8-8��0 |
��3�����ݡ����ʾ�����;����;�������ʡ��ش��������⡣
A��ϡ��Ũ����ʱ��Ϊʲô���ɽ�ˮ����Ũ��������Ҫ������ԭ��
B������������ᶼ��ʹ��ɫʯ����Һ��죬Ϊʲô��
C��д����ϡ�����ˮ������Ҫ�ɷ���̼��ƣ��Ļ�ѧ����ʽ��
��4����֧�Թ��зֱ�װ��������ɫ���壬���Ƿֱ����Ȼ��ơ��������ơ�̼����е�һ�֣�ֻҪ���������� ���ܽ�����һ���Լ��������
��5��ʵ���������ʱijͬѧ������������ʵ�飺������ͭ��ϡ���ᷴӦ�����ռ���Һ���̪��Һ��Ӧ��ʵ�������ͬѧ���ַ�Һ��������ɫ����������д������ɫ�������ɵķ�Ӧ�Ļ�ѧ����ʽ�� ��
 ��6����ͼ��Ũ�����Լ�ƿ�ϱ�ǩ�IJ������ݡ���ش�
��6����ͼ��Ũ�����Լ�ƿ�ϱ�ǩ�IJ������ݡ���ش�
A����Ҫ����9����ϡ����120g����Ҫ����Ũ���� g��
B��Ũ����ʹ��һ��ʱ�����������������С��ԭ����ʲô��
C��ij�������������ڿ����г��ڷ���һ��ʱ��ֱ��ʣ����ù���ȡ10 g������������Ϊ9%�����������ٲ�������Ϊֹ����������������Ϊ2.2g���Լ���δ���ʵ��������Ƶ�����Ϊ���ٿˣ�
��1��������������ֻ�Ϳ����е�ˮ�����йص���______
A�������ռ�Ҫ�ܷ���
B����ʯ�ҳ����ڿ����з��û����
C��Ũ����¶���ڿ����л����ء���ϡ
D������Ʒ���ڳ�ʪ�����л�����
��2��������һЩʳ��Ľ���pH������θ�������˱Ƚ����˵�ʳ����______
| ѡ�� | A | B | C | D |
| ���� | ���� | �ݲ� | ���� | ������ |
| pH | 2.9��3.5 | 3.0��4.0 | 3.5��4.5 | 6.8-8.0 |
A��ϡ��Ũ����ʱ��Ϊʲô���ɽ�ˮ����Ũ��������Ҫ������ԭ��
B������������ᶼ��ʹ��ɫʯ����Һ��죬Ϊʲô��
C��д����ϡ�����ˮ������Ҫ�ɷ���̼��ƣ��Ļ�ѧ����ʽ��
��4����֧�Թ��зֱ�װ��������ɫ���壬���Ƿֱ����Ȼ��ơ��������ơ�̼����е�һ�֣�ֻҪ����������______���ܽ�����һ���Լ�����������ᣨ����������ѧʽHCl��������36%�ܶ�l��18g/cm3
��5��ʵ���������ʱijͬѧ������������ʵ�飺������ͭ��ϡ���ᷴӦ�����ռ���Һ���̪��Һ��Ӧ��ʵ�������ͬѧ���ַ�Һ��������ɫ����������д������ɫ�������ɵķ�Ӧ�Ļ�ѧ����ʽ��______��
��6����ͼ��Ũ�����Լ�ƿ�ϱ�ǩ�IJ������ݣ���ش�
A����Ҫ����9%��ϡ����120g����Ҫ����Ũ����______g��
B��Ũ����ʹ��һ��ʱ�����������������С��ԭ����ʲô��
C��ij�������������ڿ����г��ڷ���һ��ʱ��ֱ��ʣ����ù���ȡ10g������������Ϊ9%�����������ٲ�������Ϊֹ����������������Ϊ2.2g���Լ���δ���ʵ��������Ƶ�����Ϊ���ٿˣ�