��Ŀ����
����Ŀ�������д����л�ѧ��������ѧ֪ʶ�ش��������⣺
(1)ijѧУʳ�õ�����ṩ��������С���ࡢ���������ͷ������������Ӿ���Ӫ���ĽǶȽ���ʳ�����Ӻ�___��ʳ��(�����)��
A ������ B ��֬ C ���� D ά����
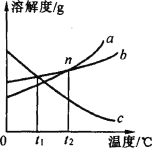
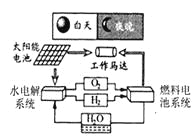
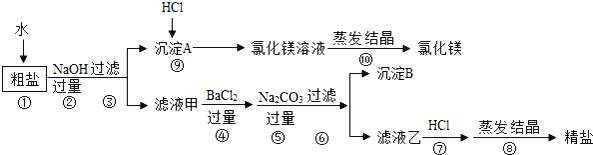
(2)��ͼ������ʾ���г��ĸ������У������л��ϳɲ��ϵ���_____(����)��
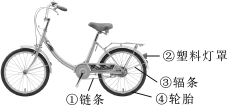
(3)��ȩ(CH3OH)�ж���������ʹ�۾�ʧ�����������������������о�֤�����ð���(NH3)�������м�ȩ�Ĺ�ҵ��ˮ����ʹ��ת����������ʣ��йط�Ӧ�Ļ�ѧ����ʽΪ5CH3OH��12O2��6NH3![]() 3X��5CO2��19H2O����X�Ļ�ѧʽΪ____��
3X��5CO2��19H2O����X�Ļ�ѧʽΪ____��
(4)����(C3H8)�Ǽ���Һ��ʯ��������Ҫ�ɷ�֮һ�����ͼ����ڿ�����ȼ�յ���������ͬ����д������ȼ�յĻ�ѧ����ʽ��_______________��
���𰸡�D �ڢ� N2 
��������
��1�������и��������ʣ�С��������࣬��������������ʡ���֬����ͷ�������������࣬��Ӧ����ά���ء�
��ѡD��
��2���������������ɽ�����Ͻ��Ƴɣ����ڽ������ϣ���̥�ɺϳ����Ƴɣ����ںϳɲ��ϣ����ϵ����������Ƴɣ����ںϳɲ��ϣ������ںϳɲ��ϵ��ǣ��ڢܣ�
��3�����������غ㶨�ɣ���ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬��ϻ�ѧ����ʽ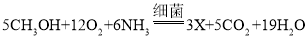 ����Ӧ����̼ԭ�ӵ���ĿΪ5����ԭ��Ϊ38����ԭ��Ϊ29����ԭ��Ϊ6���������У�̼ԭ��Ϊ5����ԭ��Ϊ38����ԭ��Ϊ29������������Ӧ����ԭ�ӵ���ĿΪ6����X�Ļ�ѧʽΪ��N2��
����Ӧ����̼ԭ�ӵ���ĿΪ5����ԭ��Ϊ38����ԭ��Ϊ29����ԭ��Ϊ6���������У�̼ԭ��Ϊ5����ԭ��Ϊ38����ԭ��Ϊ29������������Ӧ����ԭ�ӵ���ĿΪ6����X�Ļ�ѧʽΪ��N2��
��4�������ڿ�����ȼ�����ɶ�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��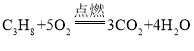 ��
��

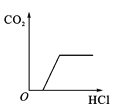
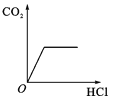
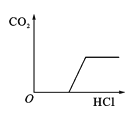
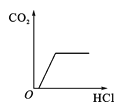
����Ŀ�������ĸ�ͼ���У�����ȷ��ӳ��Ӧ�仯��ϵ����
|
|
|
|
A����һ���������������������� | B���һ��������ˮ | Cľ̿�ڸ����º���������Ӧ | D��������ȫ��ͬ�Ĺ���������Һ�ֱ���ȡ���� |
A.AB.BC.CD.D