��Ŀ����
�����ǵ���Ҫ�ɷ���̼��ƣ�ij��ȤС��Ϊ�˲ⶨ��������CaCO3�ĺ�������ȡ15g�����ǣ����飬�����ձ��У�Ȼ�������м���80gijŨ�ȵ�ϡ���ᣬʹ֮��ַ�Ӧ���������г�CaCO3��������ɷֶ�������ˮ���Ҳ���ϡ���ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������ˮ�����Ļӷ��������е���Ӧ���е�B��ʱ����������պ������˼�������һ�룬�Լ��㣨����������1λС����

��1������CO2������Ϊ g��
��2���ü�������CaCO3������������
��3������ϡ���������ʵ�����������

��1������CO2������Ϊ g��
��2���ü�������CaCO3������������
��3������ϡ���������ʵ�����������
��1��4.4g��2��66.7%��3��18.3%
��1��̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ɵĶ�����̼��ɢ�������У��ձ���ǰ����ٵ�������Ϊ������̼���������ʲ���CO2������Ϊ��95g-90.6g=4.4g
��2�����ݶ�����̼���������̼��Ƶ�����
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 4.4g
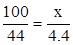 x=10g
x=10g
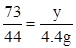 y=7.3g
y=7.3g
������CaCO3����������Ϊ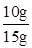 ��100%=66.7%
��100%=66.7%
��3��ϡ���������ʵ���������Ϊ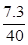 ��100%��18.3%
��100%��18.3%
��2�����ݶ�����̼���������̼��Ƶ�����
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 44
x y 4.4g
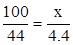 x=10g
x=10g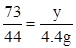 y=7.3g
y=7.3g������CaCO3����������Ϊ
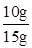 ��100%=66.7%
��100%=66.7%��3��ϡ���������ʵ���������Ϊ
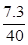 ��100%��18.3%
��100%��18.3%
��ϰ��ϵ�д�
�����Ŀ



