��Ŀ����
 ��֪�ס��ҷֱ���ϡ���������������Һ�е�һ�֣�ij��ѧ��ȤС���ͬѧ��̽��������Һ�����кͷ�Ӧʱ����÷�Ӧ��������Һ���ȱ仯�����������ͼ��ʾ���Ը���Ҫ�ش��������⣺
��֪�ס��ҷֱ���ϡ���������������Һ�е�һ�֣�ij��ѧ��ȤС���ͬѧ��̽��������Һ�����кͷ�Ӧʱ����÷�Ӧ��������Һ���ȱ仯�����������ͼ��ʾ���Ը���Ҫ�ش��������⣺
��1�����ݴ˱仯���ߣ�����Ϊ����Һ�е�������________����д��ѧʽ��
��2�����������Һ��������5gʱ��������ɫ��̪��Һ����Һ��________ɫ����ʱ����Һ�е�����Ϊ________����д��ѧʽ��
��3�����������Һ��������15gʱ�����ʱ��Һ�м��������Լ��������������ʵ���������________����д���и������ţ���
��̼���Ʒ�ĩ�����������Ȼ�����Һ
������������Һ��������ʯ����Һ
��4����ǡ����ȫ��Ӧʱ��Һ�����ʵ���������д����Ӧ������̣�
�⣺��1����ͼ���֪��ԭ��ҺpH��7������ǰ�ϡ�����������������Һ�У����ԣ�����Һ�е�������H2SO4��
��2����ͼ���֪�����������Һ��������5gʱ��pH��7����Һ����������δ��ȫ��Ӧ������������Dz������ģ���Һ�е�������NaOH��Na2SO4��
��3����ͼ���֪�����������Һ��������15gʱ��pH��7����Һ������������ȫ��Ӧ������������ǹ����ģ�������̼���Ʒ�ĩ��Ӧ���������ɣ����Ȼ�����Һ��Ӧ�а�ɫ�������ɣ���ʹʯ����Һ��죬������������Һ��Ӧû�����Ե�����
��4����ͼ���֪�����������Һ��������10gʱ��������������������ǡ����ȫ��Ӧ��
�����ȫ��Ӧʱ��Һ�����ʵ�����ΪX
2NaOH+H2SO4 =2H2O+Na2SO4
98 142
10g��19.6% X
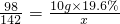 ��ã�X=3.04
��ã�X=3.04
�ʴ�Ϊ����1��H2SO4����2����ɫ��NaOH��Na2SO4����3���ۣ���4��ǡ����ȫ��Ӧʱ��Һ�����ʵ�������3.04g��
��������1������ͼ���֪��ԭ��ҺpH��7������ǰ�ϡ�����������������Һ�У�
��2������ͼ���֪�����������Һ��������5gʱ��pH��7����Һ����������δ��ȫ��Ӧ������������Dz������ģ��ݴ˷�����Һ�е����ʣ�
��3������ͼ���֪�����������Һ��������15gʱ��pH��7����Һ������������ȫ��Ӧ������������ǹ����ģ��ݴ˷����йص����⣻
��4������ͼ���֪�����������Һ��������10gʱ��������������������ǡ����ȫ��Ӧ���ٸ��ݱ�ǩ�е����ݼ���Ӧ�ķ���ʽ���ɼ�����Һ�����ʵ�����
������������ͼ�����ʽ����������кͷ�Ӧʱ��ҺpH�ı仯���Լ����ʼ��������ϵ����ɴ��⣬�����������е�֪ʶ���У��йصļ���Ҫȷ��
��2����ͼ���֪�����������Һ��������5gʱ��pH��7����Һ����������δ��ȫ��Ӧ������������Dz������ģ���Һ�е�������NaOH��Na2SO4��
��3����ͼ���֪�����������Һ��������15gʱ��pH��7����Һ������������ȫ��Ӧ������������ǹ����ģ�������̼���Ʒ�ĩ��Ӧ���������ɣ����Ȼ�����Һ��Ӧ�а�ɫ�������ɣ���ʹʯ����Һ��죬������������Һ��Ӧû�����Ե�����
��4����ͼ���֪�����������Һ��������10gʱ��������������������ǡ����ȫ��Ӧ��
�����ȫ��Ӧʱ��Һ�����ʵ�����ΪX
2NaOH+H2SO4 =2H2O+Na2SO4
98 142
10g��19.6% X
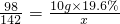 ��ã�X=3.04
��ã�X=3.04�ʴ�Ϊ����1��H2SO4����2����ɫ��NaOH��Na2SO4����3���ۣ���4��ǡ����ȫ��Ӧʱ��Һ�����ʵ�������3.04g��
��������1������ͼ���֪��ԭ��ҺpH��7������ǰ�ϡ�����������������Һ�У�
��2������ͼ���֪�����������Һ��������5gʱ��pH��7����Һ����������δ��ȫ��Ӧ������������Dz������ģ��ݴ˷�����Һ�е����ʣ�
��3������ͼ���֪�����������Һ��������15gʱ��pH��7����Һ������������ȫ��Ӧ������������ǹ����ģ��ݴ˷����йص����⣻
��4������ͼ���֪�����������Һ��������10gʱ��������������������ǡ����ȫ��Ӧ���ٸ��ݱ�ǩ�е����ݼ���Ӧ�ķ���ʽ���ɼ�����Һ�����ʵ�����
������������ͼ�����ʽ����������кͷ�Ӧʱ��ҺpH�ı仯���Լ����ʼ��������ϵ����ɴ��⣬�����������е�֪ʶ���У��йصļ���Ҫȷ��

��ϰ��ϵ�д�
�����Ŀ
 23����ͼ��ʾ����ʢ��10mLϡ�ļ���Һ�����е���������ɫʯ���Լ����У���������Һʱ����ҺpH�ı仯���ߣ���֪�ס��ҷֱ���������Һ������������Һ�е�һ�֣���������ش����⣺
23����ͼ��ʾ����ʢ��10mLϡ�ļ���Һ�����е���������ɫʯ���Լ����У���������Һʱ����ҺpH�ı仯���ߣ���֪�ס��ҷֱ���������Һ������������Һ�е�һ�֣���������ش����⣺ ��֪�ס��ҷֱ���ϡ���������������Һ�е�һ�֣�ij��ѧ��ȤС���ͬѧ��̽��������Һ�����кͷ�Ӧʱ����÷�Ӧ��������Һ���ȱ仯�����������ͼ��ʾ���Ը���Ҫ�ش��������⣺
��֪�ס��ҷֱ���ϡ���������������Һ�е�һ�֣�ij��ѧ��ȤС���ͬѧ��̽��������Һ�����кͷ�Ӧʱ����÷�Ӧ��������Һ���ȱ仯�����������ͼ��ʾ���Ը���Ҫ�ش��������⣺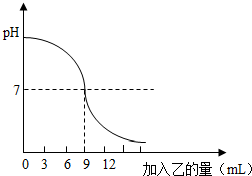 ��ͼ��ʾ����ʢ��lOmLϡ�ļ���Һ�����е���������ɫʯ���Լ����У���������Һʱ����ҺpH�ı仯���ߣ���֪�ס��ҷֱ���������Һ������������Һ�е�һ�֣���������ش����⣺
��ͼ��ʾ����ʢ��lOmLϡ�ļ���Һ�����е���������ɫʯ���Լ����У���������Һʱ����ҺpH�ı仯���ߣ���֪�ס��ҷֱ���������Һ������������Һ�е�һ�֣���������ش����⣺