��Ŀ����
��2011?��̨����ģ�����������Dz���ȱ�ٵ�����Ʒ����1��������ʹ�õļӷ������еġ�������ָ���ԭ�ӡ���Ԫ�ء�����
��2�������е�Ħ�����Ǽ�ϸС��̼��Ʒ�ĩ������������������ͼ��ʾ��
�ٲ����Ļ�����Ӧ����Ϊ��
�ڲ��������Ӧ�Ļ�ѧ����ʽΪ��
��3����ͼ����˵�����У����������ƣ�Na2PO3F������Ԫ����ɣ��������е��������ƣ���Է�������Ϊ144������������Ϊ�����������λС������
| ����˵���� ��Ҫ���Գɷ֣����������� ��Na2PO3F�� ����������100g �� ����100mg �� Ч��Ԥ������ |
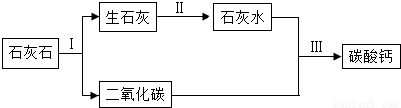
���𰸡��������������е�֪ʶ���з������ӷ������еķ�ָ���Ƿ�Ԫ�أ�̼��Ƹ����ֽܷ����������ƺͶ�����̼������������ˮ���������������ƣ����ݵ��������ƵĻ�ѧʽ���ɿ��������Ԫ��Ϊ���֣���������˵���������õ��������Ƶ�����������
����⣺��1���ӷ������еķ�ָ���Ƿ�Ԫ�أ����Ա����Ϊ��Ԫ�أ�
��2����̼��Ƹ����ֽܷ����������ƺͶ�����̼���˷�ӦΪ�ֽⷴӦ�����Ա����Ϊ���ֽⷴӦ��
������������ˮ���������������ƣ����Ա����Ϊ��CaO+H2O=Ca��0H��2��
��3�����ݵ��������ƵĻ�ѧʽ��Na2PO3F�����ơ��ס�����������Ԫ����ɣ���������˵���飬����100mg��Ҫ���������Ƶ�����Ϊx��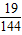 =
=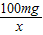 �����x=757.9mg�����Ե��������Ƶ���������Ϊ����
�����x=757.9mg�����Ե��������Ƶ���������Ϊ����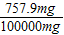 ×100%=0.76%�����Ա����Ϊ��0.76%��
×100%=0.76%�����Ա����Ϊ��0.76%��
���������⿼����̼��ơ��������Լ���������֮���ת������ɴ��⣬�����������е�֪ʶ���У�
����⣺��1���ӷ������еķ�ָ���Ƿ�Ԫ�أ����Ա����Ϊ��Ԫ�أ�
��2����̼��Ƹ����ֽܷ����������ƺͶ�����̼���˷�ӦΪ�ֽⷴӦ�����Ա����Ϊ���ֽⷴӦ��
������������ˮ���������������ƣ����Ա����Ϊ��CaO+H2O=Ca��0H��2��
��3�����ݵ��������ƵĻ�ѧʽ��Na2PO3F�����ơ��ס�����������Ԫ����ɣ���������˵���飬����100mg��Ҫ���������Ƶ�����Ϊx��
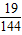 =
=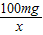 �����x=757.9mg�����Ե��������Ƶ���������Ϊ����
�����x=757.9mg�����Ե��������Ƶ���������Ϊ����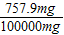 ×100%=0.76%�����Ա����Ϊ��0.76%��
×100%=0.76%�����Ա����Ϊ��0.76%�����������⿼����̼��ơ��������Լ���������֮���ת������ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
�����Ŀ
��2011?��̨����ģ��С������ʵ���ң�����ʵ��������һƿ���ڷ��õ��������ƹ��壬���Ƕ����Ƿ���ʲ�������Ȥ������ͬѧ��������ƿ�������̽����
�������ϣ�a��̼������Һ�Լ��ԣ��Ȼ�����Һ�����ԣ�
b��̼������Һ���Ȼ�����Һ���Է�����Ӧ��Na2CO3+CaCl2=CaCO3��+2NaCl��
�������룺
����1������û�б���
����2�����岿�ֱ���
����3��______
��Ʒ�����
ͬѧ�Ǿ������ۣ���Ϊ����������������������һ��������ԭ����______��
��������������ԭ����______��ͬѧ����������˷�����������ʵ�飮
ʵ��̽����
������3��ȷ����ʵ�������Ӧ����______��
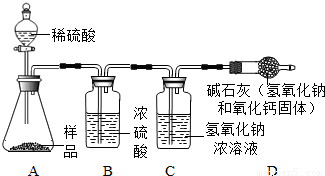
����̽����ͬѧ�Ǽ���̽�����������ƹ�����Ʒ��̼���Ƶ�������������������װ�ý�����ʵ�飮
����Ϊ������Ҫ���������______������ĸ��ţ���
a��������Ʒ������ b����ʯ��ʵ��ǰ�������
c��Ũ����ʵ��ǰ������� d������������Һʵ��ǰ�������
e������ϡ���������
ʵ�鷴˼�������������ݽ��м��㷢�ֽ����ʵ��ƫС��ԭ�������______��
�������ϣ�a��̼������Һ�Լ��ԣ��Ȼ�����Һ�����ԣ�
b��̼������Һ���Ȼ�����Һ���Է�����Ӧ��Na2CO3+CaCl2=CaCO3��+2NaCl��
�������룺
����1������û�б���
����2�����岿�ֱ���
����3��______
��Ʒ�����
| ʵ����� | ʵ������ | ʵ����� | |
| ����һ | ȡ������������Թ��У��μӼ���ϡ���� | ���������� | ����1��ȷ |
| ������ | ȡ������������Թ��м�ˮ�ܽ⣬�μ���������ʯ��ˮ | ������ɫ���� | ����2��ȷ |
| ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ | ��Һ��Ϊ��ɫ |
��������������ԭ����______��ͬѧ����������˷�����������ʵ�飮
ʵ��̽����
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������������Թ��м�ˮ�ܽ⣬�μ�����______ | ������ɫ���� | ����2��ȷ |
| ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ | ______ |
����̽����ͬѧ�Ǽ���̽�����������ƹ�����Ʒ��̼���Ƶ�������������������װ�ý�����ʵ�飮
����Ϊ������Ҫ���������______������ĸ��ţ���
a��������Ʒ������ b����ʯ��ʵ��ǰ�������
c��Ũ����ʵ��ǰ������� d������������Һʵ��ǰ�������
e������ϡ���������
ʵ�鷴˼�������������ݽ��м��㷢�ֽ����ʵ��ƫС��ԭ�������______��

 2NaOH+Cl2��+H2�������Ƶ�40g�������ƣ�ͬʱ�ɵõ������������Ƕ��٣�
2NaOH+Cl2��+H2�������Ƶ�40g�������ƣ�ͬʱ�ɵõ������������Ƕ��٣�