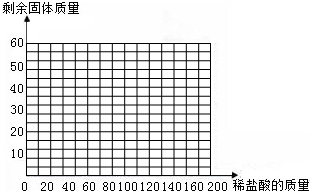
��Ŀ����
��3�֣���һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2����֪SiO2�Ȳ�����ˮҲ�������ᷴӦ�����ֽ�һ������ʯ��ʯ��Ʒ�����ձ��У��ٽ�100gϡ�����4�μ����ձ��У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ���������/g | 25 | 25 | 25 | 25 |
| ��ַ�Ӧ��ʣ���������/g | 7 | 4 | 2 | M |
��2����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3�����ɶ�����̼����������
2g 80% 3.52g
����������������⡿���������֪��ÿ����25gϡ���ᣬ���彫��Ӧ��3g���������η�Ӧ����廹��2g��˵����ʱ�����е�2g�����Ƕ������裬�������Ӧ�ˡ��ʣ�
��1������������M��ֵΪ2g ��
��2���������ݱ��ɷ���25��ϡ������ȫ��ӦӦ����̼���7 g - 4 g ="3" g����˷�Ӧǰʯ��ʯ��Ʒ������ӦΪ7 g +3 g ="10" g��
��Ʒ��CaCO3����������Ϊ�� ��100% ��80%
��100% ��80%
��3���裺���ɶ�����̼������Ϊx��
CaCO3 + 2HCl  CaCl2 + H2O + CO2��
CaCl2 + H2O + CO2��
100 44
8g x 
x=3.52g
�����ɶ�����̼������Ϊ3.52g��
���㣺���ݻ�ѧ����ʽ���㡣
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢�𡣱����з������ݣ��ó�ÿ����25gϡ���ᣬ���彫��Ӧ��3g��2g�����Ƕ��������ǽ���Ĺؼ���

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�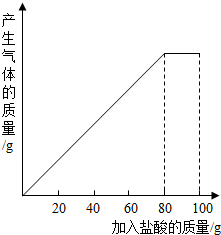 ��һ��ʯ��ʯ��Ʒ����Ҫ�ɷ���CaCO3������С��ͬѧ��100gϡ�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪���ʲ������ᷴӦҲ������ˮ�����õ����²������ݺ�ͼ������ϸ�۲����б����ͼ����㣺
��һ��ʯ��ʯ��Ʒ����Ҫ�ɷ���CaCO3������С��ͬѧ��100gϡ�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪���ʲ������ᷴӦҲ������ˮ�����õ����²������ݺ�ͼ������ϸ�۲����б����ͼ����㣺| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��2��ʯ��ʯ��Ʒ��CaCO3��������Ϊ���٣��������ȷ��0.1%��
��3��10%��CaCl2��Һ����Ϊ·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ���ҷ�ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
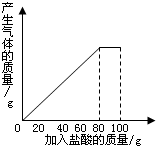 ��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��1����2�μ��������aΪ
��2��ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊ���٣����������������ȱ�ʾ��
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| �������������/g | 20 | 20 | 20 | 20 | 20 |
| ʣ����������/g | 30 | 25 | 20 | 15 | 15 |
| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| �������������/g | 20 | 20 | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 | 15 | 15 |
��1����2�μ��������aΪ
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
��4������35gʯ��ʯ��Ʒ�м��������������ʣ�����������仯��ϵ��ʾ��ͼ���ڡ��������ͼ����ͼ��