��Ŀ����
����Ŀ��ijͬѧ��������������̽�������˶����ռ�һ����ƿ����������������ʵ�飺�ٳ��ڷ��ã���˫����������ƿ����ϣ��۽�����ƿ��ƿ�ڳ��¡�����ʵ����ȷ������ƿ���β��䣬ʵ�������������������ʱ��仯����������Ϊ��ͼ�е�MN�Ρ�NP�κ�PQ�Ρ�

��1�����ܹ�˵�������Ӳ����˶���������______��������ţ�
A .MN�� B .NP�� C .PQ��
��2��MN�κ�NP����Ƚϣ�˵��_________________________��
��3������ʵ��Ľ��У�����������������������ԼΪ���֮һʱ���ٱ仯��ԭ����_____��
���𰸡�A �¶�Խ�ߣ������˶��ٶ�Խ�� ƿ��ȫ���ǿ���,�������������������ԼΪ21��
��������
��1���ܹ�˵�������Ӳ����˶���������MN�Σ�������Ϊ���ڷ��ã���������������ϼ�С���������Dz����˶��ģ������������˶���ƿ�⣻˫����������ƿ����ϣ�ƿ���¶����ߣ�����֮������ᵼ�²������������ݳ�������˵�������Dz����˶��ģ�������ƿ��ƿ�ڳ��£������������������ö��½�������˵�������Dz����˶��ġ�
��2��MN�κ�NP����Ƚϣ�NP���������������С�ĽϿ죬˵���¶�Խ�ߣ������˶���Խ�졣
��3������ʵ��Ľ��У�����������������������ԼΪ21%ʱ�������ٱ仯��������Ϊƿ��ȫ���ǿ������������������������ԼΪ21%��

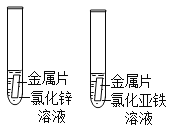
����Ŀ����ͭ��ͭ��п�ĺϽ�ij��ѧ��ȤС���ͬѧ���ⶨij��ͭ��Ʒ��ͭ�����������������ǻ�ͭ�е��������ʣ�������������ǵ�̽�����̡����� 10g ��ĩ״��ͭ��Ʒ�����ձ��У� �� 60g ijŨ�ȵ�ϡ��������μӵ����У�ÿ�γ�ַ�Ӧ�ⶨ����������������ʵ��������
�±���
��һ�� | �ڶ��� | ������ | |
����ϡ�����������g�� | 20 | 20 | 20 |
����������������g�� | 0.04 | m | 0.02 |
����
��1��m ����ֵ_____��
��2��10g ��ͭ�� 60g ϡ�����ַ�Ӧ����������������_____��
��3���˻�ͭ��Ʒ��ͭ������������_____��
��4����������Һ�����������������٣�____����д���������,���������� 1 λС����