��Ŀ����
2005��1��1���𣬸��ػ������Ž���ʵ��������Ⱦʵʩ�ϸ�Ļ�����ܣ�ijУʵ��������50kg�������Ʒ�Һ����У��ij������������ϡ�������кͺ��ŷţ��������ҺpH=7ʱ���кͺ���Һ��������Ϊ100kg����÷�Ӧ�����Һ�����ʵ���������Ϊ7.1%����
��1����Ӧ���ɵ������Ƶ������Ƕ��٣�
��2�����õ�ϡ���������ʵ���������Ϊ���٣�
��3����Уʵ���ҵ��������Ʒ�Һ�����ʵ����������Ƕ��٣�
��1����Ӧ���ɵ������Ƶ������Ƕ��٣�
��2�����õ�ϡ���������ʵ���������Ϊ���٣�
��3����Уʵ���ҵ��������Ʒ�Һ�����ʵ����������Ƕ��٣�
��1��100kg��7.1%=7.1kg��
��2������뷴Ӧ��H2SO4������Ϊx�����뷴Ӧ��NaOH������Ϊy��
2NaOH+H2SO4=Na2SO4+2H2O
80 98 142
y x 7.1kg
��98��142=x��7.1kg��80��142=y��7.1kg��
��֮�ã�x=4.9kg��y=4kg��
��100%=9.8%��
��3��
�� 100%=8%��
�𣺣�1����Ӧ���ɵ������Ƶ�������7.1kg��
��2�����õ�ϡ���������ʵ���������Ϊ9.8%��
��3����Уʵ���ҵ��������Ʒ�Һ�����ʵ�����������8%��
��2������뷴Ӧ��H2SO4������Ϊx�����뷴Ӧ��NaOH������Ϊy��
2NaOH+H2SO4=Na2SO4+2H2O
80 98 142
y x 7.1kg
��98��142=x��7.1kg��80��142=y��7.1kg��
��֮�ã�x=4.9kg��y=4kg��
| 4.9kg |
| 100kg-50kg |
��3��
| 4kg |
| 50kg |
�𣺣�1����Ӧ���ɵ������Ƶ�������7.1kg��
��2�����õ�ϡ���������ʵ���������Ϊ9.8%��
��3����Уʵ���ҵ��������Ʒ�Һ�����ʵ�����������8%��

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
�����Ŀ
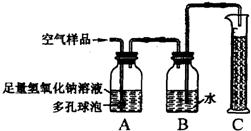 22���ݱ�������2005��1��1���𣬹���ʡ���ػ������Ž���ʵ��������Ⱦʵʩ�ϸ�Ļ�����ܣ�����ѧУ��ѧʵ����Ҫ�ŷųɷָ��ӵ���Ⱦ�����Ҳ����Ϊ������ܷ�Χ��ijУ��ѧ��ȤС���ͬѧ�ڼ�ʵ�����н����������ֱ���̼��������Ӧ��ʵ���Ϊ�˽��ʵ�����������Կ����ɷ���ɵ�Ӱ�죬�������������ʵ��װ�ý���ʵ�飨ͼ�ж�����ݵ������ǣ�������������Һ�ĽӴ������ʹ��Ӧ��ֽ��У���
22���ݱ�������2005��1��1���𣬹���ʡ���ػ������Ž���ʵ��������Ⱦʵʩ�ϸ�Ļ�����ܣ�����ѧУ��ѧʵ����Ҫ�ŷųɷָ��ӵ���Ⱦ�����Ҳ����Ϊ������ܷ�Χ��ijУ��ѧ��ȤС���ͬѧ�ڼ�ʵ�����н����������ֱ���̼��������Ӧ��ʵ���Ϊ�˽��ʵ�����������Կ����ɷ���ɵ�Ӱ�죬�������������ʵ��װ�ý���ʵ�飨ͼ�ж�����ݵ������ǣ�������������Һ�ĽӴ������ʹ��Ӧ��ֽ��У���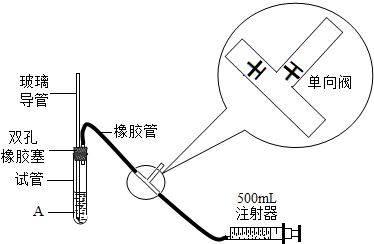
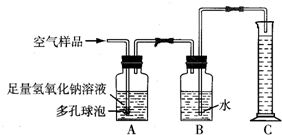 A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���
A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���