��Ŀ����
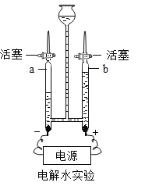
����Ŀ����7�֣�ij��ȤС��Ϊ����֤�����غ㶨�ɣ����ձ���ʢ��20g������������Ϊ20%������������Һ�Թ���װ��20g����ͭ��Һ����ͼ��ʾ��������ƽ�ϳ�������ʱ��ƽƽ�⡣Ȼ���Թ���ҩƷ�����ձ��ڣ��Թܲ�ȡ������ǡ����ȫ��Ӧ���Ը���Ҫ�ش��������⣺

��1��д��ʵ������й۲쵽��һ��ʵ������________________________________________________��
��2����Ӧ��������ƽָ���__________ ������ƫ������ƫ������������ƫת��֮һ����
��3���Լ���ǡ����ȫ��Ӧʱ���ò�������Һ������������ȷ��0.1g��
���𰸡���1��������ɫ��������2��������ƫת��
��3��������������ͭ������Ϊx
2NaOH + CuSO4 = Na2SO4 + Cu(OH)2��
80 98
20g.20% x
![]() =
=![]() x=4.9g
x=4.9g
��Ӧ�����ò�������Һ������Ϊ20g +20g-4.9g=35.1g
�𣺷�Ӧ�����ò�������Һ������Ϊ 35.1g
��������
�����������1������������Һ������ͭ��Һ��Ӧ��������ɫ������ ��2����������ҩƷû�лӷ��ԣ����ɵij���Ҳ���ձ��У����Է�Ӧ��������ƽָ�벻����ƫת����3�����������غ㶨�ɣ���Ӧ��������Һ���������Ƿ�Ӧǰ������Һ������֮�ͼ�ȥ����������ͭ���������������ԣ��ؼ���������ɵ�������ͭ�����������ݸ������������Ƶ����������������ͭ��������