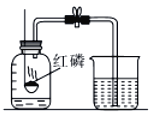
��Ŀ����
����Ŀ����������������Ҫ��Ӧԭ������:
��1��NaCl + NH3 + CO2 + H2O =NaHCO3 + NH4C1
��2��2NaHCO3![]() Na2CO3 +CO2 +H2O����������Ϣ���й����ⲻ��ȷ���ǣ�������
Na2CO3 +CO2 +H2O����������Ϣ���й����ⲻ��ȷ���ǣ�������
A. ��1�������������ʣ����Һ��ֻ��һ������
B. ����ˮ��ʳ��ˮ�������ն�����̼
C. ��������NaHCO3���ܽ�ȱ�NH4C1��С
D. ������������ҺΪNaHCO3�ı�����Һ
���𰸡�A
��������
A����1�������������ʣ����Һ�к���NH4Cl��NaCl����ѡ��˵��������B������ˮ�Լ��ԣ����������̼��Ӧ������ˮ��ʳ��ˮ�������ն�����̼����ѡ��˵����ȷ��C����������NaHCO3���ܽ�ȱ�NH4Cl��С����ѡ��˵����ȷ��D��������������ҺΪNaHCO3�ı�����Һ����ѡ��˵����ȷ����ѡA��

����Ŀ����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ����ѧʵ��Ϳ�ѧ̽���벻��ʵ��װ�á�
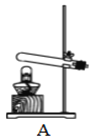
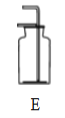
��ȡ����ij���װ�� |
| | | |
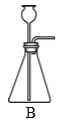
�ռ�����ij���װ�� |
| | | |
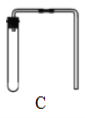
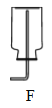
��������ij���װ�� |
|
| ||
(1)��ȡ����ʵ��ʱ������Ҫ�Է�����װ�ý��������Լ�飬ȷ��װ�ò�©����װ��C�����Լ��ķ����ǣ�_________ (���Ⱥ�˳������ĸ )�������ܿ������ݣ����ֺ�������һ���ȶ���ˮ���������������á�
[A]������ס�Թ� [B]������һ�˽���ˮ��
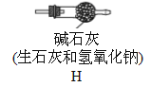
�����ϣ�Ũ���������հ���������ǿ��ˮ�ԣ���ʯ��������ˮ�ֺͶ�����̼��
(2)ʵ���ҳ����Ȼ�����������ƵĹ��������������ȡ��������֪�����ܶȱȿ���С����������ˮ��������������ռ�װ�ÿ�ѡ��A��F�е�_______________װ�á�
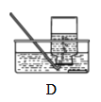
(3)ʵ�����ÿ�״ʯ��ʯ��ϡ�����Ʊ�CO2����Ӧ�ķ��ű���ʽΪ______���Ʊ����ռ������Ķ�����̼����ѡװ����ȷ������˳����_____ (��װ�ñ�� )��