��Ŀ����
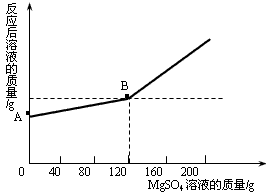
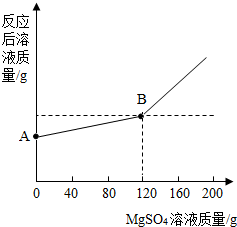
��֪�����ƺ�ˮ�ܷ������з�Ӧ��2Na+2H2O=2NaOH+H2�� ��ȡ������4.6 gͶ�뵽100 gˮ�У���ַ�Ӧ����ȴ�����£�20�棩���õ�һ�ֲ�������Һ����������Һ����ε���MgSO4��Һ,ʵ������Һ�������������MgSO4��Һ��������ϵ������ͼ��ʾ����������ش��������⣺
�Ž����ƺ�ˮ��ַ�Ӧ����������������Ϊ g��
��ͼ��A����������ʾ����Һ�������� g��
��ͨ���������������120gMgSO4��Һʱ��������Һ�����ʵ����������Ƕ��٣�����������ȷ��0.1%��
��ͼ��A����������ʾ����Һ�������� g��
��ͨ���������������120gMgSO4��Һʱ��������Һ�����ʵ����������Ƕ��٣�����������ȷ��0.1%��
�� 0.2
�� 104.4
�⣺������NaOH������Ϊx��
2Na+2H2O===2NaOH + H2��
46 80
4.6g x
46��80 = 4.6g��x x = 8g
�� ��������Na2SO4������Ϊy������ Mg(OH)2������Ϊz��
MgSO4+2NaOH===Na2SO4+Mg(OH)2��
80 142 58
8g y z
80��142 = 8g��y y = 14.2g
80��58 = 8 g��z z = 5.8g
��Ӧ����Һ��������Ϊ��4.6g+100g+120g-5.8g-0.2g=218.6g
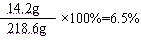
�𣺷�Ӧ�����Һ�����ʵ���������Ϊ6.5%
�� 104.4
�⣺������NaOH������Ϊx��
2Na+2H2O===2NaOH + H2��
46 80
4.6g x
46��80 = 4.6g��x x = 8g
�� ��������Na2SO4������Ϊy������ Mg(OH)2������Ϊz��
MgSO4+2NaOH===Na2SO4+Mg(OH)2��
80 142 58
8g y z
80��142 = 8g��y y = 14.2g
80��58 = 8 g��z z = 5.8g
��Ӧ����Һ��������Ϊ��4.6g+100g+120g-5.8g-0.2g=218.6g
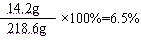
�𣺷�Ӧ�����Һ�����ʵ���������Ϊ6.5%

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
 ��֪�����ƺ�ˮ�ܷ������з�Ӧ��2Na+2H2O=2NaOH+H2��
��֪�����ƺ�ˮ�ܷ������з�Ӧ��2Na+2H2O=2NaOH+H2��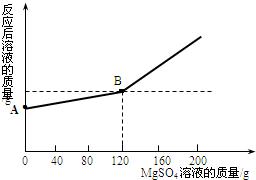 20����֪�����ƺ�ˮ�ܷ�����Ӧ��2Na+2H2O=2NaOH+H2��
20����֪�����ƺ�ˮ�ܷ�����Ӧ��2Na+2H2O=2NaOH+H2��