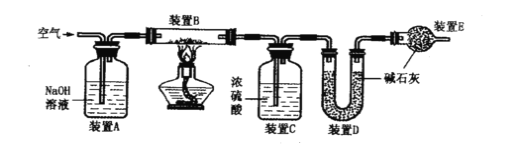
��Ŀ����
����Ŀ��Ϊ�˲ⶨij��ĩ״��ͭ��ͭ��п�Ͻ���Ʒ��ͭ������������ijͬѧ��ȡm g�Ļ�ͭ��Ʒ�����ձ��У���ȡ40gϡ������Ĵμ����ձ��У�����ַ�Ӧ��ʵ���������£�
��һ�� | �ڶ��� | ������ | ���Ĵ� | |
����ϡ����������g�� | 10 | 10 | 10 | 10 |
ʣ������������g�� | 9.10 | 8.45 | 7.80 | 7.80 |
��1��ͨ������ȷ��m��ֵΪ__________��
��2��ͭп�Ͻ���ͭ����������Ϊ����_________����д����1������2��������̣�
��3��������ϡ���������ʵ���������__________����д��������̣�
���𰸡� 9.75 80% 7.3%
�����������⿼�����������������뻯ѧ��Ӧ����ʽ���ϵļ��㡣����������ʵ�����ݷ���ÿ�μ���10gϡ�������ʣ���������ı仯����ȷ�μӷ�Ӧ���ǹ����е�п����������Ӧ���е���������ǽ������Ĺؼ���
��1�����ݽ�����Կ�֪��ͭ�����������ϡ���ᷢ����Ӧ��������Ӧ����п�����ٵĹ�����������Dzμӷ�Ӧ��п�����������ڵ����μ����������������٣�˵���ڶ��μ����ϡ������ȫ�μӷ�Ӧ���ڶ��η�Ӧ����п������=9.10g-8.45g=0.65g���ɴ˿�֪ÿ����10g���������п������Ϊ8.45g-7.80g=0.65g��������ʱ���ٹ��������ǡ����0.65g���ҵ��Ĵκ͵�����ʣ�����������ȣ�˵��������30g����ǡ���ܷ�Ӧ����Ʒ�е�п���������ĵ�п�������ǣ�0.65g��3=1.95g��ʣ�������δ�μӷ�Ӧ��ͭ������m��ֵ��1.95g+7.80g=9.75g��
��2��ͭп�Ͻ���ͭ����������=![]() ��100%=80%
��100%=80%
��3�����ݽ�����Կ�֪��ͭ�����������ϡ���ᷢ����Ӧ��������Ӧ����п�����ٵĹ�����������Dzμӷ�Ӧ��п����������������1���ķ�����֪��ÿ10g���ᷴӦ����п��������0.65g��
����10gϡ���������ʵ�����Ϊx��
Zn+2HCl�TZnCl2+H2��
65 73
0.65g x
![]() x=0.73g
x=0.73g
ϡ���������ʵ���������=![]() ��100%=7.3%��
��100%=7.3%��
����1��ͨ������ȷ��m��ֵΪ9.75g��
��2��ͭп�Ͻ���ͭ����������Ϊ����80%��
��3��������ϡ���������ʵ���������7.3%��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ʵ�鼼��ѵ���У�С��ͬѧ������ͭ��ϡ�����ַ�Ӧ��ķ�Һ�м���һ����������������Һ����������������С��ͬѧ����ʦ��ָ���¶�����ͭ��ϡ�����ַ�Ӧ��ķ�Һ������ʵ�飺���ֱ�ȡ50g��Һ���������������Ũ�ȵ�����������Һ������ʵ���������ͼ�����£�

ʵ������ | ��һ�� | �ڶ��� | ������ |
��������������Һ����/g | 50 | 100 | 80 |
��������������/g | 0.98 | 2.94 | 2.94 |
�����������Ϣ�ش��������⣺
(1)����ͭ��ĩ��ϡ���ᷴӦ������Ϊ___________________��
(2)��Һ�е�������_______________(�ѧʽ) ���������Һ������ͭ��������������____________��(д���������)
(3)�����������У�ֻ��һ����������������Һ���Һǡ����ȫ��Ӧ������ͼ��a����ֵΪ____________��