��Ŀ����
�ڡ���¹���̷��¼�֮ǰ�������̷�����Щ���������������̷ۡ��¼��������̷�������ΪIJȡ������������̷��е����ʵĺ���������ʳ�������̷۵��ڶ�Ӥ������Ӫ����������������Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ������ֲ��á��Ƕ�����ֽ����еĵ����ʡ���ԭ���ǰѵ������еĵ�Ԫ����ȫת��Ϊ��������ѧʽΪNH3��������ϡ�������հ�������Ӧ�Ļ�ѧ����ʽΪ��2 NH3+H2SO4= (NH4)2 SO4����ȡ���̷���Ʒ100g���á��Ƕ�����ֽ����еĵ����ʣ������İ�����7.5g������������Ϊ19.6%��ϡ����ǡ����ȫ���ա�
���㲢�ش��������⣺
��1�����������������Ƕ��٣�����������ȷ��0.01����ͬ��
��2������Ԫ�ص��������٣�
��3�����̷��е��ĺ����Ƿ�ﵽ�˹��ҹ涨�ı������̷��е����ʵĺ������ұ�Ϊ��ÿ100gӤ���̷��к�12g~25g�������ʺ���Ԫ�ص���������Ϊ16%��
��1��0.51g ��3�֣���2��0.42g ��2�֣� ��3������� ��1��

��ϰ��ϵ�д�
�����Ŀ
2008��9�£��й�������¹�̷��¼�������ʳ��������Ⱦ�̷۵�Ӥ����������ʯ��������ԭ�����̷��к��������谷{C3N6H6[C3N3��NH2��3]}�������谷�۸���ˣ�����������ţ�̺��̷������������谷����Ҫ������ð�䵰���ʣ���ߺ���������ƭ�ʼ첿�ź������ߣ���Ʒ���̷��е����ʺ���Ϊ15��20%���������к�����ƽ��Ϊ16%�����й��������谷��˵���д�����ǣ�������
| A�������谷������Ԫ����� | B������������к��������ġ�������ָԪ�� | C��ÿ�������谷�����к���6��̼ԭ�ӡ�12����ԭ�Ӻ�12����ԭ�� | D��ţ�̺��̷������������谷������ߺ�������Ҳ����ߵ����ʵĺ��� |
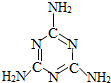 ��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�
��ȫ���ġ���¹�̷ۡ��¼��е���������������谷�����ӽṹ��ͼ���������谷��һ����Ҫ�Ļ���ԭ�ϣ������京�����ߣ���Ŀǰ��������ͨ�á�������궨ţ���е����ʺ���������һЩ�������˾ͽ�������̷����ԡ���ߡ��̷۵�Ʒ�ʣ�