��Ŀ����
AΪ��ɫ���壬A��C��D��Y���������E�ǵ��ʣ�F��GΪ���������ʣ�����B��E��G���ڵ��ʣ���Ӧ����������ҵ�е���Ҫ��Ӧ����ͼ������֮����ת����ϵ����ش�
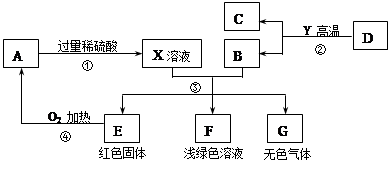
��1��X��Һ�������е�����Ϊ ������A�Ļ�ѧʽΪ________��F��Һ��dz��ɫ�����������е�������__________��
��2��д����Ӧ����������ɫ����Ļ�ѧ����ʽ ��
��3��ָ������G��A�ڼ��������·����Ļ�ѧ��Ӧ�Ļ�����Ӧ���� ��
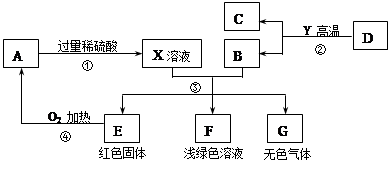
��1��X��Һ�������е�����Ϊ ������A�Ļ�ѧʽΪ________��F��Һ��dz��ɫ�����������е�������__________��
��2��д����Ӧ����������ɫ����Ļ�ѧ����ʽ ��
��3��ָ������G��A�ڼ��������·����Ļ�ѧ��Ӧ�Ļ�����Ӧ���� ��
��1��CuSO4��H2SO4 ��CuO �� FeSO4 ��2�� Fe + H2SO4 = FeSO4 + H2�� ��3�� �û���Ӧ
���������������������֪ EΪ����ͭΪ���ͻ�ƿڣ�AΪ����ͭ����Ϊ����������ԣ�1��X��Һ�������е�����Ϊ���������ͭ������A�Ļ�ѧʽΪCuO��F��Һ��dz��ɫ�����������е��������������������ԣ�2�� д����Ӧ����������ɫ����Ļ�ѧ����ʽFe + H2SO4 = FeSO4 + H2������3������GΪ������AΪ����ͭ���ʷ�Ӧ������Ϊ�û���Ӧ��

��ϰ��ϵ�д�
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ
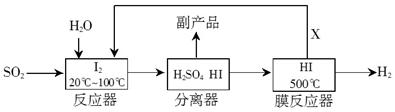
 H2+I2
H2+I2