��Ŀ����
(6��) ˮ����Һ�������������������������ʮ����Ҫ�����á�
��1����ͼʵ���У����Թ�1����������Ϊ6mLʱ���Թ�2���������ԼΪ mL��������Ӧ�Ļ�ѧ����ʽΪ________��

��2��ˮ���������Ƹ�����Һ���������Һ�е�����Ϊ________��
��3��ũҵ�����������ʵ���������Ϊ10% ~ 20%��NaCl��Һ��ѡ�֡��ֽ�300 g 25%��NaCl��Һϡ��Ϊ15%��NaCl��Һ����Ҫ��ˮ������Ϊ________g��
��4�������±��ش����⡣
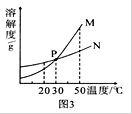
�� 60 ��ʱ���������ֱ�ʢ��50 g NaCl��NH4Cl���ձ��У�������100 g��ˮ������ܽ��Ϊ������Һ����________��Һ��
�� ������ʵ���������ձ���������40�档����˵���У���ȷ����________������ĸ��ţ���
A�������ձ��е���Һ��Ϊ������Һ
B�������ձ�����Һ�����������ܲ���
C�������ձ�����Һ��������С��һ����
D�������ձ������ʵ�����������һ����С
��1����ͼʵ���У����Թ�1����������Ϊ6mLʱ���Թ�2���������ԼΪ mL��������Ӧ�Ļ�ѧ����ʽΪ________��

��2��ˮ���������Ƹ�����Һ���������Һ�е�����Ϊ________��
��3��ũҵ�����������ʵ���������Ϊ10% ~ 20%��NaCl��Һ��ѡ�֡��ֽ�300 g 25%��NaCl��Һϡ��Ϊ15%��NaCl��Һ����Ҫ��ˮ������Ϊ________g��
��4�������±��ش����⡣
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� /g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 |
| NH4Cl | 29.4 | 37.2 | 45.8 | 55.2 | 65.6 | 77.3 | |
�� ������ʵ���������ձ���������40�档����˵���У���ȷ����________������ĸ��ţ���
A�������ձ��е���Һ��Ϊ������Һ
B�������ձ�����Һ�����������ܲ���
C�������ձ�����Һ��������С��һ����
D�������ձ������ʵ�����������һ����С
��1��3 2H2Oͨ��2H2��+ O2�� ��2��KNO3
��3��200 ��4���� NaCl �� AD
��3��200 ��4���� NaCl �� AD
�����������1�����ˮ��ʵ���У��ھ����������⣬�����һ�������Դ�������������������������Դ������������������������������������������2�������Ե��Թ�1����������Ϊ6mLʱ���Թ�2���������ԼΪ3mL��������Ӧ�Ļ�ѧ����ʽΪ��2H2Oͨ��2H2��+ O2��
��2����Һ�����ƣ����ʵ��ܼ���Һ�����ܼ�Ϊˮʱ����ʡ�Բ�˵���������Һ�е�����ΪKNO3
��3���ֽ�300 g 25%��NaCl��Һϡ��Ϊ15%��NaCl��Һ��ϡ���������ʵ��������䣬���Կ�����ˮ������Ϊx������ʽ��300g��25%=(300g+x)��15%,x=200g
��4���ٸ��ݱ��е��ܽ����ֵ��60 ��ʱ��NaCl���ܽ��Ϊ37.3g��NH4Cl���ܽ��Ϊ55.2g�����������ֱ�ʢ��50 g NaCl��NH4Cl���ձ��У�������100 g��ˮ������ܽ��Ϊ������Һ����NaCl��Һ���ڵ����µ�40�棬��ʱNaCl���ܽ��Ϊ36.6g��NH4Cl���ܽ��Ϊ45.8g�����Զ���Ϊ������Һ��A�������ձ��е���Һ��Ϊ������Һ����ȷ��B��NaCl��NH4Cl���о��������������ձ�����Һ�������������˱仯������C��NaCl��NH4Cl���ܽ�����¶ȵı仯���Ʋ�ͬ�������ձ�����Һ��������С�IJ�һ��������D����Ϊ���º�NaCl��NH4Cl���о������������ܼ����䣬���������ձ������ʵ�����������һ����С����ȷ��ѡAD

��ϰ��ϵ�д�
�����Ŀ