��Ŀ����
������ijС��ͬѧѧϰ����������Һ��ϡ���ᷴӦʱ��ʵ¼��
��ʢ������������Һ���ձ��У�����һ֧�¶ȼƲ�����Һ���¶ȡ��ý�ͷ�ι���ȡ10%��ϡ������μ���ʢ������������Һ���ձ��С�
��̽��ʵ���������仯��
��ʵ����̣�С�����֣�����ϡ����IJ��ϵ��룬��Һ���¶������ߺ͡�����Ϊ��Һ���¶������ߺ͵�ԭ���ǣ� ����2�֣�
��̽����Ӧ�յ����⣺
ʵ���У������¶��б仯�⣬û���κ����Ե�ʵ������ʵ�������С���ʵ�����õ���Һ��pH��7��Ҳ����ϡ�����ڲ�֪�������Ѿ�����������ʲô����֤���˷�Ӧ������ȫ�����أ�
С��˵����ʵ���������Һ�м��뼸����ɫ��̪��Һ������Һ��ɫû�б仯����Ӧ������ȫ���У�
С��˵��������������Һ��Ԥ�ȵ��뼸����ɫ��̪��Һ��Ȼ���ټ�ϡ���ᣬ����Һ��ɫ�պñ����ɫ����Ӧ������ȫ���У�
С��˵���������������ƹ����м�ϡ���ᣬ���۲쵽��������ȫ����ʧ����Ӧ������ȫ���С�
����Ϊ���ǵ�˵���У���ȷ���� ��������
����2�֣�
�ǹ���ʵ���е�ϸ�ں����������
��ʵ���У�ϡ��������ý�ͷ�ι���εμӣ���������Ŀ���� ��
��ʵ������У�Ҫ�ò��������Ͻ��裬��������Ŀ���� ��
��С��������ʵ����������ⷢ�������ݳ��֣�����Ϊԭ���� ��
�ȣ�4�֣�С��Ϊ�˽�һ���о���ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����
����Ʒ���������Ƶ�����������
�ƺ��������Ʒ�Ӧ�������������
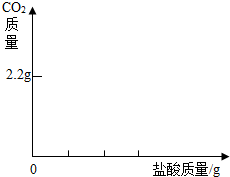
������ͼ�л������������ʾ������̼������
�������ʾ����������Ĺ�ϵͼ��
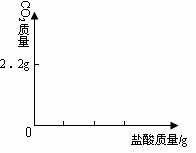 |
�������������ӣ��кͷ�Ӧ�ų�����������Һ�¶�������������������ȴ�������ã���Ӧֹͣ����ɢʧ��
��С�� С�ܵ�˵��������������ɫ��̪��Һ�����ԡ�������Һ������ɫ С���˵�������������������ƹ���Ҳ������ˮ����˵��С����Ϊ��ȷ�����ɣ�
�� �� ��ֹϡ�������
�� ʹ��Ӧ���
�� ����������Һ�к���̼����
�Ƚ⣺�������Ʒ��̼���Ƶ�����Ϊx����̼���Ʒ�Ӧ�����������Ϊy
Na2CO3 +2HCl === 2NaCl + H2O+CO2��
106 73 44
x y��10% 2.2g
106��x=73����y��10%��=44��2.2g ��1�֣�
x=5.3g y=36.5g ��2�֣�
 |

 ������ijС��ͬѧѧϰ����������Һ��ϡ���ᷴӦʱ��ʵ¼��
������ijС��ͬѧѧϰ����������Һ��ϡ���ᷴӦʱ��ʵ¼��