��Ŀ����
 ������ijС��ͬѧѧϰ����������Һ��ϡ���ᷴӦʱ��ʵ¼��
������ijС��ͬѧѧϰ����������Һ��ϡ���ᷴӦʱ��ʵ¼����ʢ������������Һ���ձ��У�����һ֧�¶ȼƲ�����Һ���¶ȣ��ý�ͷ�ι���ȡ10%��ϡ������μ���ʢ������������Һ���ձ��У�
��1��̽��ʵ���������仯��
��ʵ����̣�С�����֣�����ϡ����IJ��ϵ��룬��Һ���¶������ߺͣ�����Ϊ��Һ���¶������ߺ͵�ԭ���ǣ�
��2��̽����Ӧ�յ����⣺
ʵ���У������¶��б仯�⣬û���κ����Ե�ʵ������ʵ�������С���ʵ�����õ���Һ��pH��7��Ҳ����ϡ�����ڲ�֪�������Ѿ�����������ʲô����֤���˷�Ӧ������ȫ�����أ�
С��˵����ʵ���������Һ�м��뼸����ɫ��̪��Һ������Һ��ɫû�б仯����Ӧ������ȫ���У�
С��˵��������������Һ��Ԥ�ȵ��뼸����ɫ��̪��Һ��Ȼ���ټ�ϡ���ᣬ����Һ��ɫ�պñ����ɫ����Ӧ������ȫ���У�
С��˵���������������ƹ����м�ϡ���ᣬ���۲쵽��������ȫ����ʧ����Ӧ������ȫ���У�
����Ϊ���ǵ�˵���У���ȷ����
��3������ʵ���е�ϸ�ں����������
��ʵ���У�ϡ��������ý�ͷ�ι���εμӣ���������Ŀ����
��ʵ������У�Ҫ�ò��������Ͻ��裬��������Ŀ����
��С��������ʵ����������ⷢ�������ݳ��֣�����Ϊԭ����
��4��С��Ϊ�˽�һ���о���ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����
����Ʒ���������Ƶ�����������
�ں��������Ʒ�Ӧ�������������
����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ��
������Ū���кͷ�Ӧ��ʵ�ʣ����е������Ӻͼ��е����������ӽ������ˮ�Ĺ��̣��кͷ�Ӧ��ʹ��Һ������Է����ʵı仯���кͷ�Ӧ�����������ı仯���������������������տ����еĶ�����̼������Ӧ����̼���ƣ����������������Ʒ�еμ�ϡ����ʱ�����ж�����̼���ɣ��������ɶ�����̼�������Ϳ����̼���Ƶ��������Ӷ�������⣮
����⣺��1�������кͷ�Ӧ�����ȣ���˸տ�ʼʱ����Һ���¶Ȼ����ߣ�����������Ĺ�������Ӧֹͣ�����ҷ�Ӧ�ų�������Ҳ��֮ɢʧ���ʴ�Ϊ�������������ӣ��кͷ�Ӧ�ų�����������Һ�¶�������������������ȴ�������ã���Ӧֹͣ����ɢʧ��
��2�����ڷ�̪��Һ�������Ի�������Һ������ɫ�������������ƹ���Ҳ��������ˮ�ģ��ʴ�Ϊ��С�ģ���������ǡ����ȫ��Ӧʱ����Һ�����ԣ���̪�ĺ�ɫ�պ���ȥ
��3�����ڸ�ʵ��̽����һ���������ǡ����ȫ��Ӧ����˼�ϡ����ʱ�˵μӣ�Ϊ��ʹ���ַ�Ӧ��Ӧ���ò��������裻��������������Һ���Ϳ����еĶ�����̼��Ӧ����˳�����̼���ƣ��ʴ�Ϊ���ٷ�ֹϡ�������
��ʹ��Ӧ���
������������Һ�к���̼����
��4������ϡ����ֻ�ܺ�̼���Ʒ�Ӧ���ɶ�����̼����˿��Ը��ݶ�����̼���������̼���Ƶ������������������ʴ�Ϊ��
�⣺�������Ʒ��̼���Ƶ�����Ϊx����̼���Ʒ�Ӧ�����������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x y��10% 2.2g
=
x=
=5.3g
=
y=
=36.5g
��Ʒ���������Ƶ���������Ϊ��
��100%�T60.2%
���8g�������Ʒ�Ӧ��������Һ������Ϊz��
NaOH+HCl=NaCl+H2O
40 36.5
8g z��10%
=
z=73g��
���������Ʒ�Ӧ��������Һ������Ϊ73g��
�𣺢���Ʒ���������Ƶ���������Ϊ60.2%
�ں��������Ʒ�Ӧ���������Ϊ��73g��
����ϡ�������Ⱥͻ����Һ�е��������Ʒ�����Ӧ��Ȼ���ٺ�̼���Ʒ�Ӧ�����ݼ������֪�������������ĵ����������Ϊ��73g��̼�������ĵ������������36.5�ˣ��ʴ�Ϊ��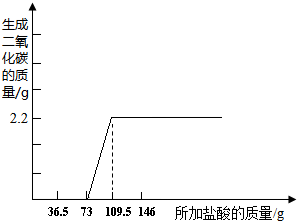
��2�����ڷ�̪��Һ�������Ի�������Һ������ɫ�������������ƹ���Ҳ��������ˮ�ģ��ʴ�Ϊ��С�ģ���������ǡ����ȫ��Ӧʱ����Һ�����ԣ���̪�ĺ�ɫ�պ���ȥ
��3�����ڸ�ʵ��̽����һ���������ǡ����ȫ��Ӧ����˼�ϡ����ʱ�˵μӣ�Ϊ��ʹ���ַ�Ӧ��Ӧ���ò��������裻��������������Һ���Ϳ����еĶ�����̼��Ӧ����˳�����̼���ƣ��ʴ�Ϊ���ٷ�ֹϡ�������
��ʹ��Ӧ���
������������Һ�к���̼����
��4������ϡ����ֻ�ܺ�̼���Ʒ�Ӧ���ɶ�����̼����˿��Ը��ݶ�����̼���������̼���Ƶ������������������ʴ�Ϊ��
�⣺�������Ʒ��̼���Ƶ�����Ϊx����̼���Ʒ�Ӧ�����������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x y��10% 2.2g
| 106 |
| 44 |
| x |
| 2.2g |
x=
| 106��2.2g |
| 44 |
| 73 |
| 44 |
| y��10% |
| 2.2g |
y=
| 73��2.2g |
| 44��10% |
��Ʒ���������Ƶ���������Ϊ��
| 13.3g-5.3g |
| 13.3g |
���8g�������Ʒ�Ӧ��������Һ������Ϊz��
NaOH+HCl=NaCl+H2O
40 36.5
8g z��10%
| 40 |
| 36.5 |
| 8g |
| z��10% |
z=73g��
���������Ʒ�Ӧ��������Һ������Ϊ73g��
�𣺢���Ʒ���������Ƶ���������Ϊ60.2%
�ں��������Ʒ�Ӧ���������Ϊ��73g��
����ϡ�������Ⱥͻ����Һ�е��������Ʒ�����Ӧ��Ȼ���ٺ�̼���Ʒ�Ӧ�����ݼ������֪�������������ĵ����������Ϊ��73g��̼�������ĵ������������36.5�ˣ��ʴ�Ϊ��

��������Ҫ�������кͷ�Ӧ����ʵ�ʺͷ�Ӧ�����а���������ܸ��ݻ�ѧ����ʽ�����йصļ��㣬����ѧ���ļ��������ͷ���������

��ϰ��ϵ�д�
 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д�
�����Ŀ