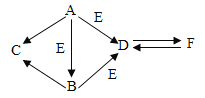
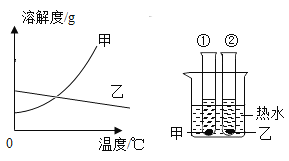
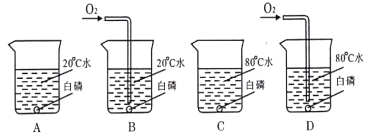
��Ŀ����
����Ŀ��С���������ʴ��������̽��������ʱ����������Ʒ����ֽ���ô�ͷ��̶��������ϣ�Ѹ��������װ����ͼ���۲쵽��Ͳ�ڵ�ˮ�ص�������������ƿ(���ݻ�Ϊ146mL)�����¶Ȼָ������£�����Ͳ��ˮ��߶Ȳ���ʱ����(��ʱƿ��������������Ϊ��)����¼��Ͳ��ʼ���������ն����Լ�����ʱ�����±���
��� | ��Ʒ��� | ��Ͳ��ʼ����/mL | ��Ͳ���ն���/mL | ����ʱ��/min |
�� | 1g���ۡ�0.2g̼��10��ˮ | 100 | 70 | Լ120 |
�� | 1g���ۡ�0.2g̼��10��ˮ������NaCl | 100 | 70 | Լ70 |
�� | _______ |
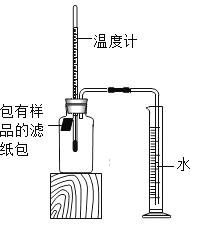
(1)ʵ��ٺ͢�˵��NaCl����_________(�����ӿ�������������)����ʴ�����ʡ�
(2)ʵ�鿪ʼ���ƿ���¶�����������˵��������ʴ������_________(��������������������)���̡�
(3)ʵ��ٺ͢���̽��̼������ʴ���ʵ�Ӱ�죬���ڱ���հ״���дʵ��۵���Ʒ��ɡ�
(4)��װ�û������ڲ��������������ĺ����������������ݼ������������������__________(��ȷ��0.1)��
���𰸡��ӿ� ���� 1g���ۡ�10��ˮ 20.5%
��������
��1��ʵ��ٺ͢ڵIJ������ʵ�����Ʒ��û���Ȼ��ƣ�ʵ�����Ʒ�к����Ȼ��ƣ���ʵ������ĵ�ʱ����������ʵ��٣�˵��NaCl���Լӿ�����ʴ�����ʣ�����ӿ졣
��2�����ƿ���¶�����������˵��������ʴ���̷��ȣ�������ȡ�
��3��ʵ��ٺ͢���̽��̼������ʴ���ʵ�Ӱ�죬�����ʵ��٣�ʵ��۵���Ʒ���Ϊ1g���ۡ�10��ˮ������1g���ۡ�10��ˮ��
��4���⣺�������������������Ϊ![]() ��100����20.5��
��100����20.5��
�𣺿������������������Ϊ20.5����

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�����Ŀ��ij��ȤС���ʯ��ʯ��Ʒ��������ʵ��ȡ12g��Ʒ�����ձ��У���100gϡ�����4�μ��뵽�ձ��У���ַ�Ӧ�����ʲ�����ˮ��Ҳ�����ᷴӦ�������ʣ������������¼���¡�����㣺
���� | 1 | 2 | 3 | 4 |
����ϡ���������/g | 25 | 25 | 25 | 25 |
ʣ����������/g | 8 | 4 | 2 | 2 |
��
��1����Ʒ��̼��Ƶ�����Ϊ_____g��
��2����4�μ���ϡ�����������Һ��CaCl2����������_______����д��������̣����ս������0.1%����
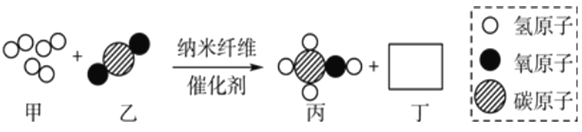
����Ŀ������ͼ��ʾ��ijУ��ѧ��ȤС���ù������⣨H2O2����Һ��MnO2�������� ��ȡ�����������������ⶨ 10g ijͭ����Ʒ������������̼����ͭ������������
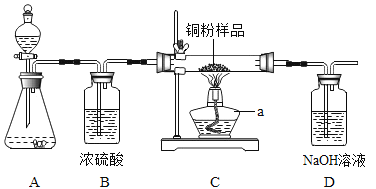
�ش��������⣺����ܰ��ʾ��2Cu+O2![]() 2CuO������������Һ�������ն�����̼��
2CuO������������Һ�������ն�����̼��
��ʵ��һ��
��1��װ��A �з����Ļ�ѧ����ʽ��__________��װ��B��������__________��
��2��ͨ��������ȫ��Ӧǰ��װ��__________�����������м����������Ʒ��ͭ������������
��3��ʵ����ϣ�С��ͬѧ����ʣ��H2O2��Һ��װ��C�������е�ʣ�����һ�����ձ��У���ͬ�д��������ݲ�����������ȷ�ϲ�����������������С����С����λͬѧ�Դ��ĸ���Ȥ������չ�������ǵ�̽��֮�á�
��������⣩
���������ʼӿ���H2O2�ķֽ����ʣ�
�����룩
����٣�ʣ������е�����ͭ�ӿ���H2O2��Һ�ķֽ⡣
����ڣ�����ͭ����H2O2�ֽ�Ĵ�����
��ʵ�����
ʵ�鷽�� | ʵ������ | ���������� |
����һ���������ǵ�ľ������ʢ��5mL5%����������Һ���Թܡ� | ������ľ������ȼ | H2O2��Һ�����²��������������٣�������ʹ�����Ǹ�ȼ�� |
���������ʢ�� 5mL5%H2O2��Һ���Թ��м��� 1g ����ͭ���壬��һ�������ǵ�ľ�������Թ��С��۲�������Ӧ�������Թ����ʣ�������___________��ϴ�ӡ�����������Աȷ�Ӧǰ������������ | �Ƶù�������Ϊ 1g | ����ٳ��������ҷ�Ӧǰ������������ȡ� |
�����۽�����
С��ͬѧ��Ϊ�����ݲ���һ������ʵ�����ó����������۾����жϲ���ڳ��������Ƿ�֧�����Ĺ۵㣿___________����Ҫ֤������ڳ���������Ϊ��Ӧ������ʵ����___________��
��4���û�ѧ��ȤС������ѹǿ����������MnO2��CuO��Fe2O3���ֽ����������У���һ��������������������ȡ�����Ĵ������������������ֻ�ʵ��̽����
��ʵ������ʵ�鷽�����£�
��.��MnO2��CuO��Fe2O3���ִ����ֱ��뺣��������Һ��ϣ������Ȼ�����Һ�Ƴɺ���������������С��ͬ�ĺ����������á�
��.ȡ30����MnO2�ĺ���������������ͼ��ʾװ�ý���ʵ�顣�������������ֱ��ظ�����ʵ�飬�õ�����ƿ��ѹǿ��ʱ��仯����������ͼ��ʾ��
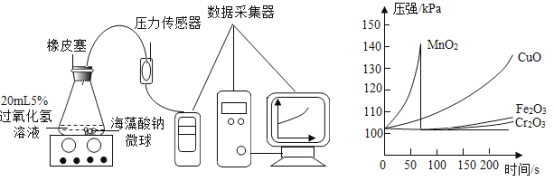
��ÿ���ظ�ʵ��ʱ��������������Ӧ��ͬ��ԭ����_________________________________��
���ú�MnO2�ĺ������������ʵ�飬60s ʱѹǿ˲����䣬��ԭ�������____________________��
�۴�ʵ�����߿�����Ч���Ϻã���Ӧ�º͵Ĵ�����______________________��
����Ŀ����һ�ܱ���������������������ˮ������һ�ֳ��пα��г��ֵ���������W����һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ��������±���ʾ��������˵���д�����ǣ� ��
���� | W | ���� | ���� | ˮ���� |
ǰ����/g | 68 | 100 | 2 | 2 |
��Ӧ������/g | X | 4 | 58 | 110 |
A. X��ֵӦΪ0
B. ��Ӧǰ��Ԫ�صĻ��ϼ۷����˱仯
C. ����W�в�����Ԫ��
D. W����������Է�������֮��Ϊ17:24