��Ŀ����
����Ŀ����ͼ��֪ʶ�Ǿ��꼶��ѧѧϰ����Ҫ֪ʶ����ش������й����⣺
��1������˵���в���ȷ���� �� ������ĸ���ţ� a����ͼ������Ԫ�� b�������κ�ˮ�ķ�Ӧһ�����кͷ�Ӧ
c��������ͼ��и�ʴ�� d����ͼ��������е��η������ֽⷴӦ
��2��Ϊ̽���ᡢ��Ļ�ѧ���ʣ�ijС��������ͼ��ʾʵ�飮 ��ʵ���ij�Թ���Ϊ��ɫ��Һ�������м���һ������ij��Һ��ɫ��ʧ��Ϊ��ɫ�����ʱ��Һ��pH7���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��ʵ��������õ�����ɫ��Һ����ɫ��Һ����ͬһ���ɾ����ձ��У��ɹ۲쵽����ɫ�������ɣ��������ԭ��
��3��ij���ڷ��õ�����������Һ�ѱ��ʣ��÷���ʽ��ʾ����ʵ�ԭ�� Ϊ֤����ƿ����������Һδ��ȫ���ʣ����������ʵ�飬�뽫������д������
ʵ����� | ���� | ���ۻ�ѧ����ʽ |
ȡ��������Ʒ���μ�������ij��Һ����ַ�Ӧ����� | �а�ɫ�������� | �йط�Ӧ�Ļ�ѧ����ʽΪ |
����Һ�еμӷ�̪��Һ | �� | ��Ʒδ��ȫ���ʣ��Ժ����������� |
��4��ȡ�ѱ��ʵ�����������Һ100g�������м���������������Ϊ7.3%��ϡ����100g��ǡ����ȫ��Ӧ�õ�������Һ���Լ������ɸ���Һ�ɵõ������������ 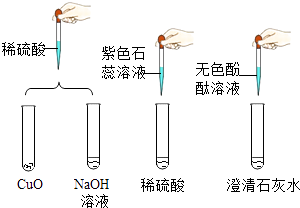
���𰸡�
��1��bd
��2���ܣ�Ca��OH��2+H2SO4�TCaSO4+2H2O���ɹ۲쵽����ɫ�������ɣ���ԭ��������ͭ��ϡ���ᷴӦ���ɵ�����ͭ��Һ�������������Ʒ�Ӧ����������ɫ������ˮ��������ͭ
��3��CaCl2+Na2CO3�TCaCO3��+2NaCl����Һ���ɫ
��4���⣺����Ԫ�������غ㣬��������Ԫ�ص������������ɵ��Ȼ�������Ԫ�ص�������
�����ɸ���Һ�ɵõ������������x
7.3%��100g�� ![]() ��100%=x��
��100%=x�� ![]() ��100%
��100%
x=11.7g
�����ɸ���Һ�ɵõ����������Ϊ11.7g
���������⣺��1��a����ͼ������Ԫ�أ�˵����ȷ��b���кͷ�Ӧ��ָ����кͣ������κ�ˮ�ķ�Ӧ��һ�����кͷ�Ӧ��������������ͼӦҲ�����κ�ˮ���������кͷ�Ӧ����˵������c��������ͼ��и�ʴ�ԣ�˵����ȷ��d�����ֽⷴӦҪ�뷢�����������б����г�����ˮ���������ɣ����Բ�������ͼ��������е��η������ֽⷴӦ����˵������ѡbd����2������Һ�ĺ�ɫ��ʧһ��������кͣ�pH��7�������Ŀ�����ṩ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ Ca��OH��2+H2SO4�TCaSO4+2H2O���ڸ������ṩ�����ʼ�ķ�Ӧ����ɫ��Һ������ͭ��Һ�����ɵ���ɫ������������ͭ��˵��������������Ʒ�Ӧʱ�������ƹ�������ɫ��Һ�к����������ƣ��������ƺ�����ͭ��Ӧ��������ɫ��������ͭ������ ���Դ��ǣ���=��Ca��OH��2+H2SO4�TCaSO4+2H2O���ڿɹ۲쵽����ɫ�������ɣ���ԭ��������ͭ��ϡ���ᷴӦ���ɵ�����ͭ��Һ�������������Ʒ�Ӧ����������ɫ������ˮ��������ͭ����3����Ҫ֤��̼���ƣ������̼���ƺ��������Ƶ����ʵIJ�ͬ��Ӧѡһ�ֺ�̼������Һ��Ӧ�а�ɫ�������ɵ����ܺ�����������Һ��Ӧ�����ʣ��ʿ�ѡ���Ȼ��ƣ�Ȼ�������Һ�Ƿ�ʼ��ԣ�
���Դ��ǣ���CaCl2+Na2CO3�TCaCO3��+2NaCl������Һ���ɫ
�����㾫����������Ҫ��������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ���ݻ�ѧ��Ӧ����ʽ�ļ�������֪ʶ�㣬��Ҫ����ע�⣺a����ƽ b������ c�����ţ������ʼ�������=ϵ������Է�������֮�Ȳ�����ȷ�����⣮

 ��У����ϵ�д�
��У����ϵ�д�