��Ŀ����
 ��֪�����ƺ�ˮ�ܷ������з�Ӧ��2Na+2H2O=2NaOH+H2��
��֪�����ƺ�ˮ�ܷ������з�Ӧ��2Na+2H2O=2NaOH+H2��
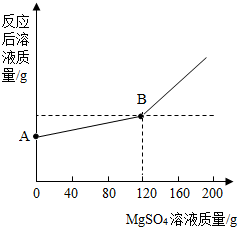
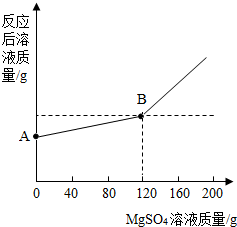
��ȡ������4.6 gͶ�뵽100 gˮ�У���ַ�Ӧ����ȴ�����£�20�棩���õ�һ�ֲ�������Һ����������Һ����ε���MgSO4��Һ��ʵ������Һ�������������MgSO4��Һ��������ϵ��������ͼ��ʾ����������ش��������⣺
��1�������ƺ�ˮ��ַ�Ӧ����������������Ϊ________g��
��2��ͼ��A����������ʾ����Һ��������________g��
��3��ͨ���������������120 g MgSO4��Һʱ��������Һ�����ʵ����������Ƕ��٣�����������ȷ��0.1%��
�⣺��1��������NaOH������Ϊx����������������Ϊw��
2Na+2H2O=2NaOH+H2��
46 80 2
4.6g x w
��46��80=4.6g��x��46��2=4.6g��w��
��֮�ã�x=8g��w=0.2g��
�ʴ�Ϊ��0.2��
��2��A����������ʾ����Һ������=4.6g+100g-0.2g=104.4g���ʴ�Ϊ��104.4��
��3������������Na2SO4������Ϊy������Mg��OH��2������Ϊz��
MgSO4+2NaOH=Na2SO4+Mg��OH��2��
80 142 58
8g y z
��80��142=8g��y��80��58=8g��z��
��֮�ã�y=14.2g��z=5.8g��
��Ӧ����Һ��������Ϊ��4.6g+100g+120g-5.8g-0.2g=218.6g��
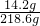 ��100%=6.5%��
��100%=6.5%��
�𣺷�Ӧ�����Һ�����ʵ���������Ϊ6.5%��
��������1�����ݽ����ƺ�ˮ��Ӧ�Ļ�ѧ����ʽ��2Na+2H2O=2NaOH+H2�����ó�������֮��������ȣ��г�����ʽ�����ɼ��������������������
��2�����������֪��ͼ��A����������ʾ����Һ���������ǽ�����4.6gͶ�뵽100gˮ�У���ַ�Ӧ����ȴ�����£�20�棩���õ�һ�ֲ�������Һ����������ˣ�A����������ʾ����Һ������=������4.6g+100gˮ-0.2g������
��3�����ݣ�1���м������NaOH����������������������þ��Ӧ�Ļ�ѧ����ʽ�����ɼ������������Na2SO4������������Mg��OH��2����������Ӧ����Һ��������=������4.6g+100gˮ+�����120gMgSO4��Һ-���ɳ���������-���ɵ�����������Ȼ�����������������=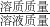 ��100%���㼴�ɣ�
��100%���㼴�ɣ�
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������
2Na+2H2O=2NaOH+H2��
46 80 2
4.6g x w
��46��80=4.6g��x��46��2=4.6g��w��
��֮�ã�x=8g��w=0.2g��
�ʴ�Ϊ��0.2��
��2��A����������ʾ����Һ������=4.6g+100g-0.2g=104.4g���ʴ�Ϊ��104.4��
��3������������Na2SO4������Ϊy������Mg��OH��2������Ϊz��
MgSO4+2NaOH=Na2SO4+Mg��OH��2��
80 142 58
8g y z
��80��142=8g��y��80��58=8g��z��
��֮�ã�y=14.2g��z=5.8g��
��Ӧ����Һ��������Ϊ��4.6g+100g+120g-5.8g-0.2g=218.6g��
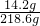 ��100%=6.5%��
��100%=6.5%���𣺷�Ӧ�����Һ�����ʵ���������Ϊ6.5%��
��������1�����ݽ����ƺ�ˮ��Ӧ�Ļ�ѧ����ʽ��2Na+2H2O=2NaOH+H2�����ó�������֮��������ȣ��г�����ʽ�����ɼ��������������������
��2�����������֪��ͼ��A����������ʾ����Һ���������ǽ�����4.6gͶ�뵽100gˮ�У���ַ�Ӧ����ȴ�����£�20�棩���õ�һ�ֲ�������Һ����������ˣ�A����������ʾ����Һ������=������4.6g+100gˮ-0.2g������
��3�����ݣ�1���м������NaOH����������������������þ��Ӧ�Ļ�ѧ����ʽ�����ɼ������������Na2SO4������������Mg��OH��2����������Ӧ����Һ��������=������4.6g+100gˮ+�����120gMgSO4��Һ-���ɳ���������-���ɵ�����������Ȼ�����������������=
 ��100%���㼴�ɣ�
��100%���㼴�ɣ�������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м����������

��ϰ��ϵ�д�
�����Ŀ
 ��֪�����ƺ�ˮ�ܷ������з�Ӧ��2Na+2H2O=2NaOH+H2��
��֪�����ƺ�ˮ�ܷ������з�Ӧ��2Na+2H2O=2NaOH+H2��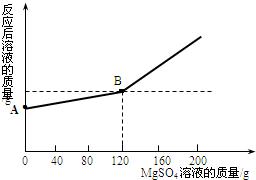 20����֪�����ƺ�ˮ�ܷ�����Ӧ��2Na+2H2O=2NaOH+H2��
20����֪�����ƺ�ˮ�ܷ�����Ӧ��2Na+2H2O=2NaOH+H2��