��Ŀ����
Ϊ�˲ⶨijͭп�Ͻ���ͭ������������ijʵ��С���ͬѧ������������ʵ��̽����
��1����һ��ͬѧ���������ʵ�飺
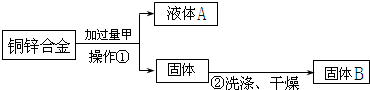
������Ϊ�������ϲ������ٳ������ù���B����������������Ͻ���ͭ����������������������ͼ�п��Կ�����������Ϊ
��2���ڶ���ͬѧ���øúϽ���ϡ���ᷴӦ�������������������������п���������Ӷ���������⣮���ǹ�����������ʵ�飬���õ����ʵ�����ݼ�¼���£�����ʵ���в���������
���ϱ����ݷ�����
��1������ȡ�Ͻ�������ϡ�����������Ϊ
��2����úϽ���ͭ��������������д��������̣�
��1����һ��ͬѧ���������ʵ�飺

������Ϊ�������ϲ������ٳ������ù���B����������������Ͻ���ͭ����������������������ͼ�п��Կ�����������Ϊ
����
����
�������ʿ�����ϡ�����ϡ����
ϡ�����ϡ����
������BΪͭ
ͭ
��С�շ���ʵ��������ȱ��ijһ���赼������ȱ���������㣬�ò����������Ͻ������
�����Ͻ������
����2���ڶ���ͬѧ���øúϽ���ϡ���ᷴӦ�������������������������п���������Ӷ���������⣮���ǹ�����������ʵ�飬���õ����ʵ�����ݼ�¼���£�����ʵ���в���������
| ��һ�� | �ڶ��� | ������ | |
| ��ȡ�Ͻ������/g | 20 | 20 | 40 |
| ���õ�ϡ���������/g | 100 | 120 | 80 |
| ���ɵ�����������/g | 0.4 | 0.4 | 0.4 |
��1������ȡ�Ͻ�������ϡ�����������Ϊ
1��4
1��4
ʱ���Ͻ��е�п��ϡ����ǡ����ȫ��Ӧ����2����úϽ���ͭ��������������д��������̣�
��������1�����ݹ��˵�ԭ�����������٣����ݺϽ���ͭ��п�����ʷ�������ļ����ʣ�Ҫ����Ͻ���ͭ�������������Ѿ�֪����ͭ��������������֪���Ͻ��������
��2��ͭ��п�Ͻ���ͭ��ϡ�����Ӧֻ��п��ϡ���ᷴӦ���ڶ��κ͵�һ����ȡ�ĺϽ�������ͬ���ڶ������õ�ϡ����ȵ�һ�ζ࣬���������ɵ�����������ͬ��˵��������ȡ�ĺϽ��е�п����ȫ��Ӧ�ˣ���������ȡ�ĺϽ���ǰ���ε�2��������Ͻ��е�п��ȫ��Ӧ��Ӧ�ò���0.8g���壬��ʵ���ϲ����������ǰ������ֻͬ��0.4g��˵�������η�Ӧ��ϡ���������п�����������ɵ���������֪�����βμӷ�Ӧ��п��ǰ������ͬ��
��2��ͭ��п�Ͻ���ͭ��ϡ�����Ӧֻ��п��ϡ���ᷴӦ���ڶ��κ͵�һ����ȡ�ĺϽ�������ͬ���ڶ������õ�ϡ����ȵ�һ�ζ࣬���������ɵ�����������ͬ��˵��������ȡ�ĺϽ��е�п����ȫ��Ӧ�ˣ���������ȡ�ĺϽ���ǰ���ε�2��������Ͻ��е�п��ȫ��Ӧ��Ӧ�ò���0.8g���壬��ʵ���ϲ����������ǰ������ֻͬ��0.4g��˵�������η�Ӧ��ϡ���������п�����������ɵ���������֪�����βμӷ�Ӧ��п��ǰ������ͬ��
����⣺��1��������ͼ�п��Կ����������ǽ���Һ�ֿ������Բ�����Ϊ���ˣ�����п�����ᷴӦ��ͭ�������ᷴӦ��Ϊ���ܵõ�ͭ��Ӧ������������Ҫ����Ͻ���ͭ�������������Ѿ�֪����ͭ��������������֪���Ͻ�����������ԣ�������ȱ��һ�����ǣ������Ͻ��������
��2���ɵ����ο�֪����0.4������ʹֻ����80��ϡ���ᣬ����20g�Ͻ��е�п��80gϡ�����ǡ����ȫ��Ӧ�����Ͻ��ϡ�����������Ϊ20g��80g=1��4ʱ��������ǡ����ȫ��Ӧ��
��2����20gͭ��п�Ͻ��к���п������Ϊx
Zn+H2SO4�TZnSO4+H2��
65 2
x 0.4g
=
��ã�X=13g
����20gͭ��п�Ͻ��к���ͭ������Ϊ20g-13g=7g
ͭ��п�Ͻ���ͭ����������Ϊ
��100%=35%
�𣺣�1�����ˣ�ϡ�����ϡ���ᣬͭ�������Ͻ����������2��1��4����ͭ��п�Ͻ���ͭ������������35%��
��2���ɵ����ο�֪����0.4������ʹֻ����80��ϡ���ᣬ����20g�Ͻ��е�п��80gϡ�����ǡ����ȫ��Ӧ�����Ͻ��ϡ�����������Ϊ20g��80g=1��4ʱ��������ǡ����ȫ��Ӧ��
��2����20gͭ��п�Ͻ��к���п������Ϊx
Zn+H2SO4�TZnSO4+H2��
65 2
x 0.4g
| 65 |
| 2 |
| X |
| 0.4g |
����20gͭ��п�Ͻ��к���ͭ������Ϊ20g-13g=7g
ͭ��п�Ͻ���ͭ����������Ϊ
| 7g |
| 20g |
�𣺣�1�����ˣ�ϡ�����ϡ���ᣬͭ�������Ͻ����������2��1��4����ͭ��п�Ͻ���ͭ������������35%��
���������⿼����Ǹ��ݻ�ѧ����ʽ���йؼ��㣬��ȷ�����������е������ǽ�����Ĺؼ���������������������ͻ�ܼ��������ڱȽ��ѵ���Ŀ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
Ϊ�˲ⶨijͭп�Ͻ���п������������ijͬѧ����һ�����ĸúϽ���ϡ���ᷴӦ������������ʵ�飬��ص�ʵ�����ݼ�¼���±���
��1�������ͭп�Ͻ���п������������
��2����������ϡ���������ʵ������������������ȷ��0.1%��
�������õ������ԭ������H-1 O-16 S-32 Cu-64 Zn-65��
| ʵ�� �������� |
��һ�� | �ڶ��� | ������ |
| ��ȡ�Ͻ������/g | 10 | 10 | 10 |
| ����ϡ���������/g | 50 | 100 | 150 |
| ʣ���������/g | 3.5 | 3.0 | 3.0 |
��2����������ϡ���������ʵ������������������ȷ��0.1%��
�������õ������ԭ������H-1 O-16 S-32 Cu-64 Zn-65��
Ϊ�˲ⶨijͭп�Ͻ���ͭ������������ȡ�Ͻ���Ʒ��ϡ���ᷴӦ������ʵ����й��������±���
��1�������ϱ��е����ݷ�������ͭп�Ͻ��е�п��ϡ�����е�HClǡ����ȫ��Ӧʱ���Ͻ���Ʒ��������ϡ���������֮��Ϊ ��
��2�������ͭп�Ͻ���ͭ������������ ��
| ʵ���� | ����ϸ���Ʒ������/g | ��ȡϡ���������/g | ��������������/g |
| 1 | 10.0 | 40.0 | 0.1 |
| 2 | 10.0 | 50.0 | 0.1 |
| 3 | 20.0 | 36.5 | 0.1 |
��2�������ͭп�Ͻ���ͭ������������
 ij�о�С��Ϊ�˲ⶨijͭп�Ͻ���ͭ������������ȡ20g�Ͻ�����ձ��У���һ������ϡ��������ɴμ����ձ��У���Ӧ���̵�������ϵ����ͼ��ʾ��x��ʾ����ϡ���������/g��y��ʾ�ձ����������ʵ�������/g�����ʣ�
ij�о�С��Ϊ�˲ⶨijͭп�Ͻ���ͭ������������ȡ20g�Ͻ�����ձ��У���һ������ϡ��������ɴμ����ձ��У���Ӧ���̵�������ϵ����ͼ��ʾ��x��ʾ����ϡ���������/g��y��ʾ�ձ����������ʵ�������/g�����ʣ� Ϊ�˲ⶨijͭп�Ͻ���п����������������������ʵ�飺ȷ��ȡ20.0g�Ͻ������в��ϼ���ϡ���ᣬ�۲쵽�������ݳ���ʵ������в�ò������������������ϡ����������ϵ��ͼ��ʾ���ش��������⣺
Ϊ�˲ⶨijͭп�Ͻ���п����������������������ʵ�飺ȷ��ȡ20.0g�Ͻ������в��ϼ���ϡ���ᣬ�۲쵽�������ݳ���ʵ������в�ò������������������ϡ����������ϵ��ͼ��ʾ���ش��������⣺