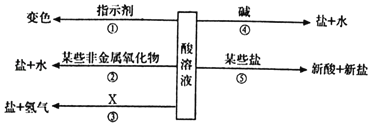
��Ŀ����
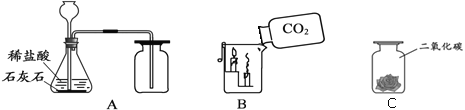
����Ŀ����ѧС��Ӧ����ͼ��ʾ��װ�ý������ʵ�飨�г�������ȥ��K1��K2��K3���رգ�װ������ʾҩƷ��������Ϊ��ʵ����ijһ״̬�µ���������֪װ���ڵ�ҩƷ���ף�ϡ�������Ϊ��ɫ���壻��Һ©����Ϊ��ɫ����Һ������ʯ��ˮ�����������Ϣ�ش����⣺��̼������Һ�ʼ��ԣ�
��1����K3�����������������г����ܿ�������ð�����ҳ��ְ�ɫ�������������е���ɫ������___�����г��ְ�ɫ���ǵ�ԭ����____�����û�ѧ����ʽ�ش�
��2���ر�K3����K2����Һ©���϶˵IJ��������ڴ�״̬��������Һ©���ڵĺ�ɫ��Һȫ�����뵽����ʱ���ر�K2������һ�����K1���ɹ۲쵽���е�������____���������������������Һ�ɺ�ɫ��Ϊ��ɫ����������Ļ�ѧ����ʽ��____�����������ʵĽǶȽ�����Һ�ɺ�ɫ��Ϊ��ɫ��ԭ����___��
���𰸡�������̼�� Ca��OH��2+CO2�TCaCO3��+H2O ������Һ�������� Na2CO3+2HCl=2NaCl+H2O+CO2�� ϡ�����������Һ������Ӧ��ʹ��Һʧȥ�˼���
��������
��1����K3�����������������г����ܿ�������ð�����ҳ��ְ�ɫ�������������е���ɫ�����Ƕ�����̼�����г��ְ�ɫ���ǵ�ԭ���ǣ�������̼�������ʯ��ˮ�У����������Ʒ�Ӧ������̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+CO2�TCaCO3��+H2O��
��2���ر�K3����K2������Һ©���ڵĺ�ɫ��Һȫ�����뵽����ʱ���ر�K2������һ������������ƺͶ�����̼��Ӧ������̼���ƺ�ˮ����������ѹǿ��С����K1���ɹ۲쵽������Һ�������У��������������������Ϊϡ�����̼���Ʒ�Ӧ�����˶�����̼����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2������Һ�ɺ�ɫ��Ϊ��ɫ������Ϊϡ�����������Һ������Ӧ��ʹ��Һʧȥ�˼��ԣ���̪��Һ�ɺ�ɫ�����ɫ��

 һ��һ��һ��ͨϵ�д�
һ��һ��һ��ͨϵ�д� �㽭֮��ѧҵˮƽ����ϵ�д�
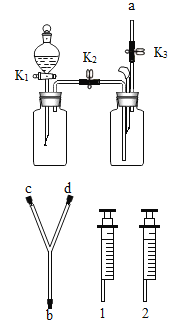
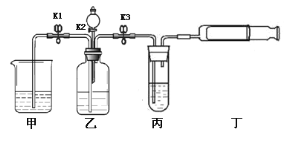
�㽭֮��ѧҵˮƽ����ϵ�д�����Ŀ��������ͼװ�ý���ʵ�顣ʵ��ǰK1��K2��K3���ѹرա�
���� װ�� | ��ʵ��1��̽��ȼ������ | ��ʵ��2��̽��CO2��NaOH��Ӧ |
| ��. A�м���������MnO2���壬��Һ©����ʢ��H2O2��Һ�� ��. B�г����ܿڴ�����ʢ�а���������ע��80������ˮ����Һ���û�¶˵��ܿڡ� | ��. A�м���������Na2CO3���壬��Һ©����ʢ��ϡH2SO4��Һ�� ��. ע����1��2�зֱ���NaOH��Һ��ϡ���� |
��1�����װ�������ԣ�����K1�رգ���K2��K3����B�м�ˮ��Һ���û�¶˵��ܿڣ�������סAƿ��ڣ�˵��װ�õ�������������õ�������________�����������������Ҳ����������á�
��2��ʵ��1�У�����H2O2��Һǰ��ˮ�еİ��ײ�ȼ�գ���K1��K2��K3����H2O2��Һ����A�У��ر�K1���۲쵽________��֤��ȼ�յ�����֮һ�ǿ�ȼ���������Ӵ���B�з�����Ӧ�Ļ�ѧ����ʽΪ________________��
��3��ʵ��2�У���K1��K2��K3����A��ע��������ϡH2SO4���ر�K1��һ��ʱ��֮����ȼ�ŵ�ľ������a�ڴ���ľ��Ϩ�𣬹ر�K2, ����ʵ�������Ŀ����______����ʱ����װ��B��a�ӿں�Y�ܵ�b�ӿ����ӣ���ע����1�е���Һ���뵽B�У��۲쵽�������Ա��Ϊ��һ��֤��������̼����������ȷʵ�����˷�Ӧ������ʵ��IJ�����������_________________��
����Ŀ����ѧʵ���Ϊͬѧ�Ǵ����˶���˼����ʵ���Ļ��ᡣ���������С�⡣
��1��ʵ����У�С����������ͼ��ʾ�ľ�ˮ����
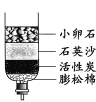
��1����װ���л���̿��������____��
��2��ʹ�ô˾�ˮ��������ˮ����_____��
A ������ B ������ C ������ D �����
��2��С���۲쵽���������ص�ȼ������
|
|
ͼ1���ղ��������� | ͼ2����������������ֽ |
��1����ȼ�ðƣ����ƾ�56%����������������������Ѹ��ȼ����������һ�������Ϩ���ˣ�������ȴ��Ȼ���������ղ�������������ԭ����_____��
��2���ô����ǵ�ľ������ؽӴ�ֽ����ë��պ�����������Һд�µ��������֣������ɣ��������л��֣��������������ֵıʼ����ӣ������ֽ�ϳ��ֳ���ë����д���������֣���������������ԭ����_____������ʾ������������������²��ȶ���