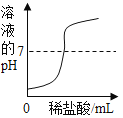
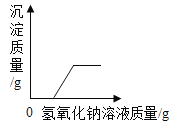
��Ŀ����
����Ŀ����ͼ��a��b��c���ֹ������ʵ��ܽ�����ߣ��ش��������⣺
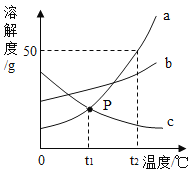
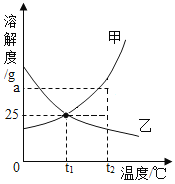
��1��t1�棬a���ܽ��_____c���ܽ�ȣ�������>������=������<����
��2��t2��ʱ����30ga���ʷ���50gˮ�г���ܽ⣬������Һ���������ܼ���������________��
��3������t2�治�䣬Ҫʹ�ӽ����͵�b��Һ��Ϊ������Һ�����ȡ�ķ�����______��
��4���ֽ�t2��ʱa��b��c�����ʵı�����Һ���µ�t1�棬������Һ��������������С��ϵΪ______��
���𰸡�= 1��2 ������b���������ˮ�� b>a>c
��������
��a��b��c���ֹ������ʵ��ܽ������ͼ��֪��a���ܽ�����¶ȵ����Ӷ�����b���ܽ�����¶ȵ����ӱ仯������c���ܽ�����¶ȵ����Ӷ���С��
��1��t1����a��c���ֹ������ʵ��ܽ�������ཻ�ڵ�p����a���ܽ��=c���ܽ�ȡ�
��2��t2��ʱ��a���ܽ��Ϊ50g����30ga���ʷ���50gˮ�г���ܽ⣬�ܽ��a������Ϊ
![]() ����������Һ���������ܼ���������
����������Һ���������ܼ���������![]()
��3��b���ܽ�����¶ȵ����ӱ仯����������t2�����䣬Ҫʹ�ӽ����͵�b��Һ��Ϊ������Һ�Ǽ�����b���������ˮ�֡�
��4��t2��ʱ��a��b��c�������ʵ��ܽ��a>b>c��a��b��c�������ʵı�����Һ���µ�t1����a�й���������b�仯������c���ܽ�ȱ�������������䣬��������Һ��������������С��ϵΪb>a>c��

 ��У����ϵ�д�
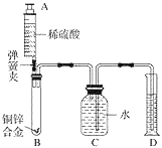
��У����ϵ�д�����Ŀ��С����������ʴ��������̽��������ʱ����������Ʒ����ֽ���ô�ͷ��̶��������ϣ�Ѹ��������װ����ͼ���۲쵽��Ͳ��ˮ�ص�������������ƿ�����ݻ�Ϊ146mL�������¶Ȼָ������£�����Ͳ��ˮ��߶Ȳ���ʱ��������ʱƿ��������������Ϊ�㣩����¼��ʼ��������Ͳ�Ķ����Լ�����ʱ�������
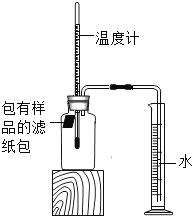
��� | ��Ʒ | ��Ͳ��ʼ ����/mL | ��Ͳ���� ����/mL | ����ʱ�� /min |
1 | 1g���ۡ�0.2g̼��10��ˮ | 100 | 70 | Լ120 |
2 | lg���ۡ�0.2g̼��10��ˮ������NaCl | 100 | 70 | Լ70 |
3 | ���� | 100 | 70 | Լ480 |
��1��ʵ��������˵��NaCl����_____����ʴ�����ʡ�
��2��ʵ�鿪ʼ���ƿ���¶�����������˵��������ʴ������_____������ȡ������ȡ������̡�
��3��ʵ�������ȡ����ֽ�����۲쵽�к���ɫ�������ɣ������ʵĻ�ѧʽ��_____��
��4����֪��̼�ܹ��ӿ����������ٶȣ�С����ͨ��ʵ����������̽��̼������ʴ���ʵ�Ӱ�죬���ڱ���հ״���дʵ��������Ʒ���_____��
��5����װ�û������ڲ��������������ĺ����������������ݼ������������������_____��С�������1λ����
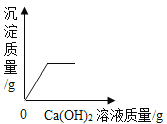
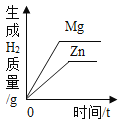
����Ŀ������ͼ������ȷ��ӳ���Ӧ�������ǣ�������
A | B | C | D |
|
|
|
|
��һ������Na2CO3��Һ����μ���Ca��OH��2��Һ | ��������Zn��Mg�ֱ����������������������ϡ���ᣨ��������Ӧ | ��NaOH��Һ����μ���ϡ���� | ��һ�������������ͭ�����Һ����εμ�����������Һ |
A. AB. BC. CD. D