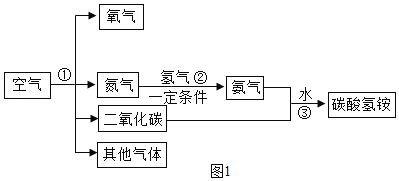
��Ŀ����
����Ŀ�������Dz����������������������ʵ�飺
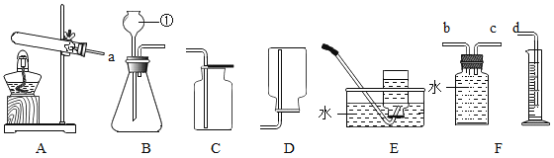
��.ʵ��С�鰴Aװ�ý���ʵ�飬д���÷�Ӧ�����ֱ���ʽ__________________��������ʵ������У��ɹ۲쵽����ı仯______________��ʵ��������ֽ��뼯��ƿ��ˮ�����С���������1/5������Ϊ������һ�����ԭ�������_________________����дһ�֣���
��.ʵ��A��ý������ȷ��ʵ��С����з�˼����С��ͨ���������ϣ�ѡ����������ͼB��ʾװ���в����������������������ȡ�óɹ���ʵ��ǰ������װ�������Եķ�����__________________��
���������ϣ������Ż�ȼ�յ��¶�Ϊ40����
��������⣩�������Լռ����������Ķ��٣�
��ʵ��������ƿ�ڿ������Ϊ230mL��ע������ˮ�����Ϊ50mL����װ�����������á�
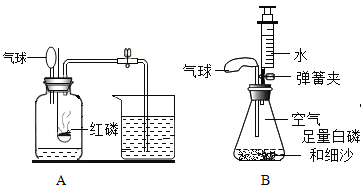
��ʵ��̽����װ��ҩƷ����ͼ��ʾ���Ӻ��������н����ɼС��Ƚ���ƿ�ײ�������ˮ�У����ܿ챻��ȼ��Ȼ����ƿ����ˮ��ȡ����
�����������
��1������ƿ�ײ�������ˮ�У�Ŀ����_________________________��
��2��������Ϩ����ƿ��ȴ�����º��ɼУ����ɹ۲쵽���������£�
��ע�����е�ˮ�Զ�������������������������ԭ���ǣ�___________________��
�ڵ�ע�����е�ˮ��ʣԼ_______mLʱֹͣ������
���𰸡�����+����![]() ���������� �ȱ����С ���ײ��㣨��װ��©����δ�ָ������¾ʹ��ɼУ� ���Ӻ�װ�ú��ɼУ��ƶ�ע�����Ļ������������� ��ȼ���� ����ȼ�պľ���ƿ�е�������ƿ��ѹǿ��С 4
���������� �ȱ����С ���ײ��㣨��װ��©����δ�ָ������¾ʹ��ɼУ� ���Ӻ�װ�ú��ɼУ��ƶ�ע�����Ļ������������� ��ȼ���� ����ȼ�պľ���ƿ�е�������ƿ��ѹǿ��С 4
��������
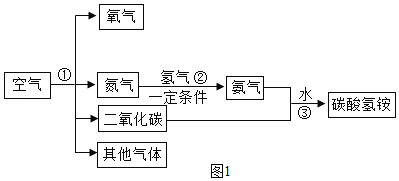
�����������������������ʵ�飺
��.ʵ��С�鰴Aװ�ý���ʵ�飬�����������ڵ�ȼ�������������������ף���Ӧ�����ֱ���ʽΪ����+����![]() ���������ס���Ӧ��ʼ���ر�ֹˮ�У�����ȼ�շų�������ƿ��ѹǿ�������������ȴ�����º�ƿ����ѹ��С�������С�����������ʵ������У��ɹ۲쵽�����ȱ����С��ʵ��������ֽ��뼯��ƿ��ˮ�����С���������1/5��������һ�����ԭ������ǣ������������û����ȴ�����¾ʹ���ֹˮ�С���װ��©���ȡ�
���������ס���Ӧ��ʼ���ر�ֹˮ�У�����ȼ�շų�������ƿ��ѹǿ�������������ȴ�����º�ƿ����ѹ��С�������С�����������ʵ������У��ɹ۲쵽�����ȱ����С��ʵ��������ֽ��뼯��ƿ��ˮ�����С���������1/5��������һ�����ԭ������ǣ������������û����ȴ�����¾ʹ���ֹˮ�С���װ��©���ȡ�
��.ʵ��A��ý������ȷ��ʵ��С��ѡ����������ͼB��ʾװ���в����������������������ȡ�óɹ���ʵ��ǰ������װ�������Եķ��������Ӻ�װ�ú��ɼУ��ƶ�ע�����Ļ������������͡�
����
��1������ƿ�ײ�������ˮ�У�Ŀ����������ˮ���¶���ȼ���ס�
��2��������Ϩ����ƿ��ȴ�����º��ɼУ����ɹ۲쵽���������£�
��ע�����е�ˮ�Զ�������������������������ԭ���ǰ���ȼ�պľ���ƿ�е�������ƿ��ѹǿ��С��
����֪��ƿ�п������Ϊ230mL���������������ԼΪ![]() ��ע������ˮ�����Ϊ50mL����ע�����е�ˮ��ʣԼ50mL -46mL=4 mLʱֹͣ������
��ע������ˮ�����Ϊ50mL����ע�����е�ˮ��ʣԼ50mL -46mL=4 mLʱֹͣ������

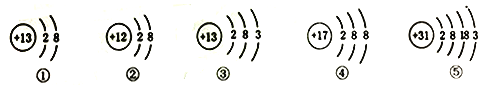
����Ŀ��������ͼ��Ԫ�����ڱ�ǰ�����ڵ�һ���֣���ͼ���ڱ���Ԫ�ص���Ϣ���������Ľṹʾ��ͼ
R | ||||
X | ||||
Y | Z |

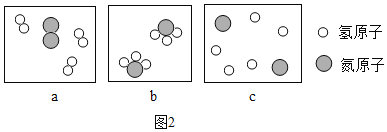
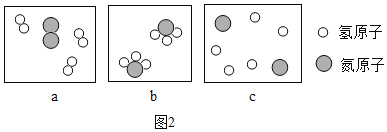
(1)R��X��Y��Z������������Ԫ����ɵĵ����У�R�Ļ�ѧ�������ȶ���R�Ļ�ѧʽ��____
(2)X��Y��Z����Ԫ���ڵؿ��еĺ�����С�����˳��������____(��Ԫ�ط���))
(3)�ٺ�Ԫ�������������γɱȽ��ȶ��Ļ�������ٵĽṹʾ��ͼΪ��_________
(4)�٢ڢ�����������ʾ��Ԫ�غ���Ԫ������ͬһ���ڵ���____(�����)
����Ŀ��ij��ȤС��ͬѧ̽��Ӱ��˫��ˮ�ֽ��ٶȵ����غ���֤�����غ㶨��ʵ�飬���ݼ�¼���£�
˫��ˮ������ | ˫��ˮ��Ũ�� | ���������� | ��ͬʱ���ڲ���O2��� | |
�� | 50.0g | 1% | MnO2 0.1g | 9 mL |
�� | 50.0g | 2% | CuO 0.1g | 12 mL |
�� | 50.0g | 2% | MnO2 0.1g | 16 mL |
��1����ʵ���У�С��ͬѧ����O2�������ѡ���ͼ�е�___________�����ţ�װ�á�
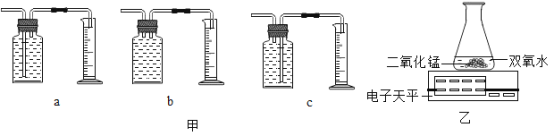
��2��ͨ��ʵ��_________��������������ѡ���С��ó����ۣ�˫��ˮ��Ũ��Խ�ֽ��ٶ�Խ�죻����Ϊ����ʵ���¼�����ݸ�С��ͬѧ���ܵõ�______���ۡ�
��3��С��ͬѧ����ͼװ�ý���ʵ�飬��Ӧ�Ļ�ѧ����ʽΪ_______���÷�Ӧ����________(�������Ӧ����)����ͨ���Ƚ�_______��Ҳ�ó�����˫��ˮŨ��Խ��Ӧ�ٶ�Խ������ʵ����ۡ�
��4��С��ͬѧ����������ƽ���ݱȻ��֮ǰ���ݼ�С�ˣ�����Ϊ�÷�Ӧ�����������غ㶨�ɡ�����Ϊ���Ŀ���_______������ȷ�����ԭ����__________��