��Ŀ����
����Ŀ��ˮ�DZ������Ȼ��Դ���������������ж��м���㷺��Ӧ�ã���ش��������⣺
��1������ˮ�ʼ��������ˮ����������д���ϸ����Ⱦ�����ø�ˮǰ�Ĵ���������_____��
��2��Ӳˮ����ˮ��������_____��
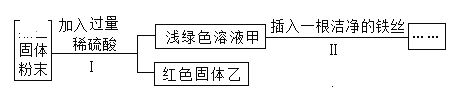
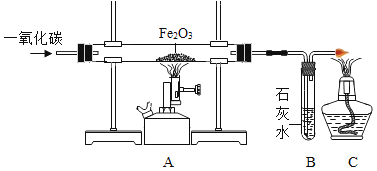
��3��������ˮ�ĺ�����ˮ������ɷ�ΪCaCO3��Mg��OH��2��ʵ���ҴӸ�ˮ������ȡ�Ȼ��ƾ������Ҫ������ͼ��
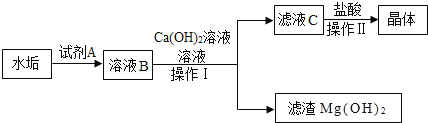
�������Լ�A��һ���ᣬ�仯ѧʽΪ_____��
��������ҺB�м���Ca��OH��2��Һ��ȥ����Ҫ������_____��д�������Ļ�ѧ��Ӧ����ʽ_____���������������_____��
����������ҺC�м����������ҪĿ����_____���������������_____��
���𰸡�������� Ӳˮ�к��д����ĸơ�þ���� HCl þ���� Ca��OH��2+MgCl2�TCaCl2+Mg��OH��2�� ���� ��ȥ�������� �����ᾧ
��������
��1��������У�����Ч��ɱ��ϸ����
��2����Ӳˮ��������Ӳˮ�к��д����ĸơ�þ���ӣ�����ˮ�н��ٻ���
��3��������ˮ����Ӧ�������Ȼ��ƺ��Ȼ�þ�����Լ�A�����ᣬ�仯ѧʽΪHCl����������������Һ�����Ȼ�þ��Ӧ����������þ������ͨ�����˳�ȥ������þ���ʳ�ȥ����Ҫ������þ���ӣ���Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+MgCl2�TCaCl2+Mg��OH��2����������������ǹ��ˣ���������þ�����������Һ�к����Ȼ��ƺ��������ƣ�����������Գ�ȥ�������ƣ�������������������ᾧ��
��1������ˮ����������д���ϸ����Ⱦ�������ڼ������ø�ˮ֮ǰ���Լ�����У�����Ч��ɱ��ϸ����
����������
��2����Ӳˮ��������Ӳˮ�к��д����ĸơ�þ���ӣ�����ˮ�н��ٻ���
���Ӳˮ�к��д����ĸơ�þ���ӣ�
��3��������ˮ����Ӧ�������Ȼ��ƺ��Ȼ�þ�����Լ�A�����ᣬ�仯ѧʽΪHCl����������������Һ�����Ȼ�þ��Ӧ����������þ������ͨ�����˳�ȥ������þ���ʳ�ȥ����Ҫ������þ���ӣ���Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+MgCl2�TCaCl2+Mg��OH��2����������������ǹ��ˣ���������þ�����������Һ�к����Ȼ��ƺ��������ƣ�����������Գ�ȥ�������ƣ�������������������ᾧ��
���������HCl��������þ���ӣ�Ca��OH��2+MgCl2�TCaCl2+Mg��OH��2�������ˣ���������ȥ�������ƣ������ᾧ��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�