��Ŀ����
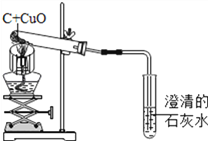
����Ŀ��̼��ԭ����ͭ��ʵ��װ�á�ij��ѧС��Ը�ʵ�鼰�����̽������:
��������
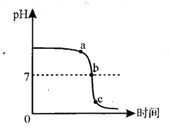
(1)д���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
(2)�ƾ��Ƽ����ֵ�Ŀ����_________________________��
(3)�տ�ʼ����ʱ������ʯ��ˮ��������ð��,������ʯ��ˮ������,ԭ����_______________��
(4)ʵ�����Ӧ��_________��(ѡ������)
A.����ƾ��� B.�������Ƴ�ʯ��ˮ. C.��������
��̽��һ���������ɷ�
��������⡿:ʵ������ǰ���ɫ���壬���ѹ۲쵽�Ϻ�ɫ���壬����ɫ������ʲô��
���������ϡ�:������ͭ(Cu2O)Ϊ��ɫ���壻Cu2O+H2SO4 =CuSO4 +Cu + H2O��
����������衿:����ɫ�����ͭ����ܺ���Cu2O
�����ʵ�顿
(5)����:ȡ��������ɫ���壬����ϡ���ᣬ�۲쵽����:___________��_____________�����ۣ�����ɫ���庬��Cu2O��
��̽�������ⶨ������Cu2O�ĺ���
(6)Ϊ�ⶨ��ɫ������������ͭ������������С��ͬѧ������������ַ�����
��������ͬѧ��ȡ10g��ɫ������˵�����ϡ������ʹ���ַ�Ӧ����_______(���������)���������ʣ���ɫ���������Ϊ8 g�����ɫ������������ͭ����������Ϊ__________��
�ҷ�����ʵ��ԭ��:Cu2O +H2![]() 2Cu +H2O,��ȡһ�������Ĺ�����Ʒ������ͼװ�ý���ʵ��(�̶�װ���ԣ���ʯ��Ϊ�����ƺ��������ƵĻ���ͨ���ⶨ��Ӧǰ��װ��d�������ﵽʵ��Ŀ�ġ�
2Cu +H2O,��ȡһ�������Ĺ�����Ʒ������ͼװ�ý���ʵ��(�̶�װ���ԣ���ʯ��Ϊ�����ƺ��������ƵĻ���ͨ���ⶨ��Ӧǰ��װ��d�������ﵽʵ��Ŀ�ġ�
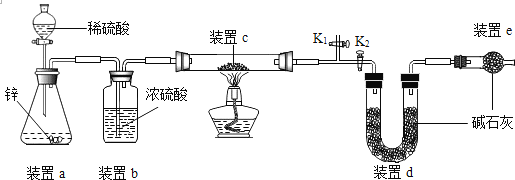
(7)װ��a����ϡ���������ϡ���ᣬԭ����_______________��
(8)������װ��c,ʵ������ƫ_______(����������С��)��ԭ����_______________��
(9)��ȼ�ƾ���ǰ�漰�IJ��ֲ������£���ȷ��˳����_____________��
�ٴ�K2,�ر�K1 �ڼ��װ�õ������� ���������� �ܹر�K2����K1��ͨ����һ��ʱ��
����չ��˼]����
(10)����ͨ���ⶨ��Ӧǰ��װ��________(����a������b������c��)�������ﵽ�ⶨ������Cu2O�ĺ���Ŀ�ġ�
(11)�ҷ�����(9)�Ģ�����ͨ����һ��ʱ������Ŀ�����ų�װ���ڵĿ�����������ܴ�����ЩӰ�죿__________��_____________(�������)
���𰸡� 2CuO +C![]() 2Cu +CO2�� �ۼ����棬����¶� ��ʱ�ݳ����������Թ��ڵĿ��������Ƕ�����̼ B ��Һ��Ϊ��ɫ �����Ϻ�ɫ���� ϴ�� 36% ����ӷ���HCl���壬��d�е����ʷ�Ӧ��Ӱ��ⶨ��� ƫ�� װ��d�����տ����е�ˮ�����Ͷ�����̼��ʹ�ⶨ����ˮ������ƫ���¼������Cu2O����ƫ�� �ۢڢܢ� c �������� ��ȼʱ������ը
2Cu +CO2�� �ۼ����棬����¶� ��ʱ�ݳ����������Թ��ڵĿ��������Ƕ�����̼ B ��Һ��Ϊ��ɫ �����Ϻ�ɫ���� ϴ�� 36% ����ӷ���HCl���壬��d�е����ʷ�Ӧ��Ӱ��ⶨ��� ƫ�� װ��d�����տ����е�ˮ�����Ͷ�����̼��ʹ�ⶨ����ˮ������ƫ���¼������Cu2O����ƫ�� �ۢڢܢ� c �������� ��ȼʱ������ը
����������1��̼��ԭ����ͭ���ɵ���ͭ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ��ʾΪ��2CuO +C![]() 2Cu +CO2������2���ƾ��Ƽ����ֵ�Ŀ���Ǿۼ����棬����¶ȣ���3���տ�ʼ����ʱ�Թ��еĿ��������ų��Թܣ�����ʼ���������Թ��ڵĿ��������Ƕ�����̼���ʳ���ʯ��ˮ������ǣ���4��ʵ�����Ӧ�Ƚ������Ƴ�ʯ��ˮ��Ϩ��ƾ��ƣ�Ŀ���Ƿ�ֹ����ʯ��ˮ�������Թ���ʹ�Թ�ը�ѣ���5���������Ͽ�֪Cu2O����ϡ���ᷴӦ������ɫ������ͭ����ͭ���ܣ�����Һ����ɫ�����ɫ�������Ϻ�ɫ���壬˵������ɫ���庬��Cu2O����6������ͭ������ܻ�մ��һЩ���������ʣ���Ҫ�����˺��ͭ����ϴ�ӣ����ɫ������ͭ������Ϊx����ôCu2O������Ϊ10g-x�����ɵ�ͭ����Ϊ8g-x�����û�ѧ����ʽ���ɼ����ͭ��������
2Cu +CO2������2���ƾ��Ƽ����ֵ�Ŀ���Ǿۼ����棬����¶ȣ���3���տ�ʼ����ʱ�Թ��еĿ��������ų��Թܣ�����ʼ���������Թ��ڵĿ��������Ƕ�����̼���ʳ���ʯ��ˮ������ǣ���4��ʵ�����Ӧ�Ƚ������Ƴ�ʯ��ˮ��Ϩ��ƾ��ƣ�Ŀ���Ƿ�ֹ����ʯ��ˮ�������Թ���ʹ�Թ�ը�ѣ���5���������Ͽ�֪Cu2O����ϡ���ᷴӦ������ɫ������ͭ����ͭ���ܣ�����Һ����ɫ�����ɫ�������Ϻ�ɫ���壬˵������ɫ���庬��Cu2O����6������ͭ������ܻ�մ��һЩ���������ʣ���Ҫ�����˺��ͭ����ϴ�ӣ����ɫ������ͭ������Ϊx����ôCu2O������Ϊ10g-x�����ɵ�ͭ����Ϊ8g-x�����û�ѧ����ʽ���ɼ����ͭ��������
Cu2O+H2SO4 =CuSO4 +Cu + H2O
144 64
10g-x 8g-x
![]() x=6.4g
x=6.4g
������ͭ����������=![]() 36%
36%
��7��������лӷ��ԣ��ӷ���HCl�����ܱ���ʯ�����գ�Ӱ��ⶨ������ʲ���ϡ�����8��������װ��e����ôd�еļ�ʯ�һ����տ����ж�����̼��ˮ���Ӷ�ʹ�ⶨ��ˮ������ƫ�ߣ��ᵼ�¼����������ͭ������ƫ�ߣ��Ӷ�����ʵ����ƫ�ߣ���9����ȼ�ƾ���Ҫ���Ӻ����������װ�õ������ԣ��ر�K2����K1��ͨ����һ��ʱ���װ���е�ˮ�����Ͷ�����̼�ų����ٴ�K2,�ر�K1����ȼ�ƾ��ƣ���˳���Ǣۢڢܢ٣���10������ͨ���ⶨ��Ӧǰ��װ��c�������ﵽʵ��Ŀ�ģ���Ӧǰ��װ��c�������Ϊ������ͭ����Ԫ�ص�������������Ԫ�ص����������������ͭ����������һ�������������������ͭ����������11����û��ͨ����һ��ʱ�䣬��ô�����Ͳ����������������ܻᷢ����ը����Ҫ��ͨ����һ��ʱ�䡣