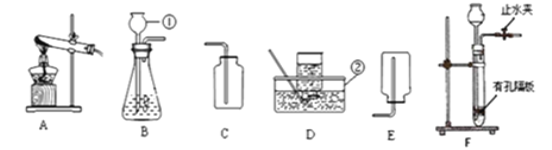
��Ŀ����
����Ŀ��ʵ���ҿ�����ͼװ��ģ�ҵ������
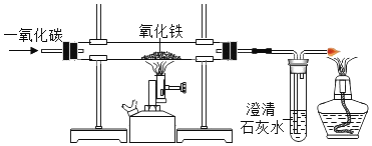
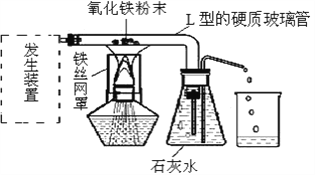
��1��һ����̼����������Fe2O3����Ӧ�Ļ�ѧ����ʽ��________��
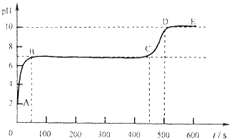
��2��ʵ��ǰ��ͨ��CO��Ŀ����_____Ӳ�ʲ������п������ձ仯��_____���Թ��г��ֵ������ǣ�_____��
��3��װ��ͼĩ�˵ĵ����ܿڷ�һյȼ�ŵľƾ��ƣ���������Ŀ����________��
��4��Ϊ���鷴Ӧ���������ɣ���������ʵ�飺����Ӧ��Ĺ���ȡ��������ϡ�����У��۲쵽������ð�����÷�Ӧ�Ļ�ѧ����ʽ��________��
���𰸡�3CO+Fe2O3![]() 2Fe+3CO2�ž�װ���ڵĿ�������ֹ����ʱ������ը����ɫ��ĩ��ɺ�ɫ������ʯ��ˮ������õ�ȼ��ȥ��һ����̼����ֹ��Ⱦ����Fe+2HCl�TFeCl2+H2��
2Fe+3CO2�ž�װ���ڵĿ�������ֹ����ʱ������ը����ɫ��ĩ��ɺ�ɫ������ʯ��ˮ������õ�ȼ��ȥ��һ����̼����ֹ��Ⱦ����Fe+2HCl�TFeCl2+H2��
��������
(1)һ����̼����������Ӧ�������Ͷ�����̼����ѧ����ʽΪ3CO+Fe2O3![]() 2Fe+3CO2��(2)һ����̼���п�ȼ�ԣ���ȼ������һ����̼���ܷ�����ը�����ʵ��ǰҪ��ͨ��һ����̼���ž�װ���ڵĿ�������ֹ����ʱ������ը������ʱ��ɫ����������ĩ��ɺ�ɫ�����ɵĶ�����̼ʹ����ʯ��ˮ�������(3)һ����̼�ж�������ֱ���ŷŵ������У����Ҫ��װ��ͼĩ�˵ĵ����ܿڷ�һյȼ�ŵľƾ��ƽ�һ����̼ȼ�ճ�ȥ����ֹ��Ⱦ������(4)���������ᷴӦ�����Ȼ���������������ѧ����ʽΪ��Fe+2HCl�TFeCl2+H2����
2Fe+3CO2��(2)һ����̼���п�ȼ�ԣ���ȼ������һ����̼���ܷ�����ը�����ʵ��ǰҪ��ͨ��һ����̼���ž�װ���ڵĿ�������ֹ����ʱ������ը������ʱ��ɫ����������ĩ��ɺ�ɫ�����ɵĶ�����̼ʹ����ʯ��ˮ�������(3)һ����̼�ж�������ֱ���ŷŵ������У����Ҫ��װ��ͼĩ�˵ĵ����ܿڷ�һյȼ�ŵľƾ��ƽ�һ����̼ȼ�ճ�ȥ����ֹ��Ⱦ������(4)���������ᷴӦ�����Ȼ���������������ѧ����ʽΪ��Fe+2HCl�TFeCl2+H2����

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�