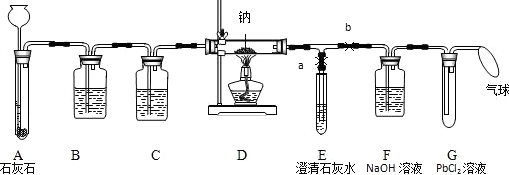
��Ŀ����
��ѧ�����������ߣ������ǵ�����ϢϢ��أ���ش����������е����⣺
��1��������Ȼ������Һ�������г�����һ�־���������ζ�������������ŵ�����ζʱ��������ע����Ȼ������Һ�������Ѿ�й©�����ŵ�������ζ����֤ʵ�˷��� �����ʣ�
��2����������Ҫ�ĵ�ζƷ������ʳ������ �������ʵĻ�ѧʽ����
��3����ʳƷ��������������������� �������ʵĻ�ѧʽ����
��4��ũҵ�������������������ļ��� �������ʵĻ�ѧʽ����
��5����ʳ�ؽ����Σ���CuSO4��BaCl2�ȣ�����ͨ���ȴ��������ķ����ⶾ������Ϊ��������
����Ҳ���Դﵽͬ����Ŀ�ģ�����ĸ����
A��ţ�� B����֭ C������ D����Ȫˮ E��ʳ��ˮ
��6������Ʒ������ʴ����д��һ�ַ�ֹ������ķ��� ��
��1��������Ȼ������Һ�������г�����һ�־���������ζ�������������ŵ�����ζʱ��������ע����Ȼ������Һ�������Ѿ�й©�����ŵ�������ζ����֤ʵ�˷���
��2����������Ҫ�ĵ�ζƷ������ʳ������
��3����ʳƷ���������������������
��4��ũҵ�������������������ļ���
��5����ʳ�ؽ����Σ���CuSO4��BaCl2�ȣ�����ͨ���ȴ��������ķ����ⶾ������Ϊ��������
����Ҳ���Դﵽͬ����Ŀ�ģ�����ĸ����
A��ţ�� B����֭ C������ D����Ȫˮ E��ʳ��ˮ
��6������Ʒ������ʴ����д��һ�ַ�ֹ������ķ���
���㣺���ӵĶ�������ӵ�����,������ʴ�������������,��ʯ�ҵ���������;,����������Ժ���;,��������ʼ�����ĺ����ⶨ,�����ж�;����Ԥ������
ר�⣺��ѧ������
��������1�����ݷ��ӵ����ʽ��н��
��2�������Ȼ��ƾ�����ζ������������Ҫ�ĵ�ζƷ���н��
��3�������������ܺ�ˮ��Ӧ�����Կ�������������н��
��4������������������ˮ�Լ��ԣ������ڸ��������������н��
��5�������ؽ������ж���ԭ�����ƻ�����ĵ����ʽṹ��ʹ֮ʧȥ�������ܽ��лش�
��6���������������������������ˮͬʱ�Ӵ����н��
��2�������Ȼ��ƾ�����ζ������������Ҫ�ĵ�ζƷ���н��
��3�������������ܺ�ˮ��Ӧ�����Կ�������������н��
��4������������������ˮ�Լ��ԣ������ڸ��������������н��
��5�������ؽ������ж���ԭ�����ƻ�����ĵ����ʽṹ��ʹ֮ʧȥ�������ܽ��лش�
��6���������������������������ˮͬʱ�Ӵ����н��
����⣺��1�����ŵ�������ζ����֤ʵ�˷��Ӳ����˶������ʣ�
��2���Ȼ��ƾ�����ζ������������Ҫ�ĵ�ζƷ��������ʳ����
��3���������ܺ�ˮ��Ӧ�����Կ�����������������������������
��4��������������ˮ�Լ��ԣ������ڸ�������������
��5���ؽ��������ƻ������ʵĽṹ��ʹ��ʧȥ�������ԣ��ȴ��������ⶾĿ���Ƿ�ֹ�ؽ������ƻ����嵰���ʣ�ֻҪ���ṩ�����ʵ����ʾ��ɣ�
A��ţ�̵���Ҫ�ɷ��ǵ����ʣ����Ժ�ţ��Ҳ�ܽⶾ����A��ȷ��
B����֭����ά���أ������������ʣ���B����
C���������Ҫ�ɷ��ǵ����ʣ����Ժȵ���Ҳ�ܽⶾ����C��ȷ��
D����Ȫˮ��Ҫ�ɷ���ˮ�������������ʣ���D����
E��ʳ��ˮ�к���ˮ�����Σ������������ʣ���E����
��ѡAC��
��6�����������������������ˮͬʱ�Ӵ������Է�ֹ������ķ�����Ϳ�͡�ˢ���ᣮ
�ʴ�Ϊ����1�������˶�����2��NaCl����3��CaO����4��Ca��OH��2����5��AC�� ��6��Ϳ�͡�ˢ���ᣮ
��2���Ȼ��ƾ�����ζ������������Ҫ�ĵ�ζƷ��������ʳ����
��3���������ܺ�ˮ��Ӧ�����Կ�����������������������������
��4��������������ˮ�Լ��ԣ������ڸ�������������
��5���ؽ��������ƻ������ʵĽṹ��ʹ��ʧȥ�������ԣ��ȴ��������ⶾĿ���Ƿ�ֹ�ؽ������ƻ����嵰���ʣ�ֻҪ���ṩ�����ʵ����ʾ��ɣ�
A��ţ�̵���Ҫ�ɷ��ǵ����ʣ����Ժ�ţ��Ҳ�ܽⶾ����A��ȷ��
B����֭����ά���أ������������ʣ���B����
C���������Ҫ�ɷ��ǵ����ʣ����Ժȵ���Ҳ�ܽⶾ����C��ȷ��
D����Ȫˮ��Ҫ�ɷ���ˮ�������������ʣ���D����
E��ʳ��ˮ�к���ˮ�����Σ������������ʣ���E����
��ѡAC��
��6�����������������������ˮͬʱ�Ӵ������Է�ֹ������ķ�����Ϳ�͡�ˢ���ᣮ
�ʴ�Ϊ����1�������˶�����2��NaCl����3��CaO����4��Ca��OH��2����5��AC�� ��6��Ϳ�͡�ˢ���ᣮ
���������⿼�����ʵ������Լ����ӵ����ʣ�Ҫ��������ǻ�ѧ����ѧ֪ʶ�ſ��ԶԴ�

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
���µļ��֡�ˮ���������ʵķ���Ƕȣ����ڴ�������ǣ�������
A�� ��е���ˮ |
B�� ����ˮ |
C�� �������Ȫˮ |
D�� ��ˮ������ |
��������ճ�������жϣ�������Һ�����Ե��ǣ�������
| A��ʯ��ˮ | B��ѩ������ |
| C������ˮ | D������ˮ |
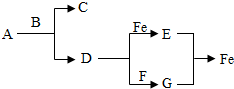 A��B��C��D��E��F���dz��л�ѧ���������ʣ�AΪһ����ɫҺ�壬BΪһ�ֺ�ɫ��ĩ��D��FΪ���ʣ��������ʾ�Ϊ�����A��C�����Ԫ����ͬ������֮��ķ�Ӧ��ͼ��ʾ�����в�����������ȥ�����ش��������⣺
A��B��C��D��E��F���dz��л�ѧ���������ʣ�AΪһ����ɫҺ�壬BΪһ�ֺ�ɫ��ĩ��D��FΪ���ʣ��������ʾ�Ϊ�����A��C�����Ԫ����ͬ������֮��ķ�Ӧ��ͼ��ʾ�����в�����������ȥ�����ش��������⣺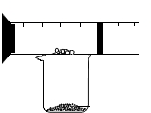 ijͬѧ����ͼ��ʾװ�ô��ԵIJⶨ���������������������ͼ���ձ��Ϸ������ܣ�Ԥ�ȹ̶��ã��в���һ�����һ����Ļ�����������ܷ��п������������ף�������Լ40�漴��ȼ�գ���ˮΪ80�棩���Ҷ˸�������ͨ��ʵ�鿪ʼǰ����������5cm����
ijͬѧ����ͼ��ʾװ�ô��ԵIJⶨ���������������������ͼ���ձ��Ϸ������ܣ�Ԥ�ȹ̶��ã��в���һ�����һ����Ļ�����������ܷ��п������������ף�������Լ40�漴��ȼ�գ���ˮΪ80�棩���Ҷ˸�������ͨ��ʵ�鿪ʼǰ����������5cm����