��Ŀ����
ij��ѧ��ȤС���ͬѧ������ľ̿��ԭ����ͭ��ʵ��ʱ����������̽����ʵ��װ����ͼ����ʾ������ľ̿���в����������ʣ���
��1���տ�ʼԤ��ʱ���Թܢ��������������ݣ���ʯ��ˮ������ǣ�ԭ����
��2���������ȣ��۲쵽�Թܢ��г���ʯ��ˮ����ǣ��Թܢ��к�ɫ��ĩ�г��ֺ�ɫ���ʣ�����д�����е�һ����ѧ����ʽ��
��3����ַ�Ӧ�۲쵽�Թܢ��е�ʣ���������������������ɫ���壬Ϊ��ȷ���ú�ɫ����ijɷ֣�ͬѧ�ǽ���������̽������������������±���
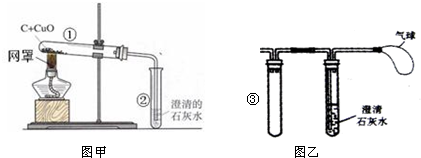
��4����ͬѧ�����ͼ����ʾ��ʵ��װ�ô��ڲ��㣬�������ͼ����ʾʵ��װ�����ͼ�����Թܢڲ��֣�ͼ���������������

��1���տ�ʼԤ��ʱ���Թܢ��������������ݣ���ʯ��ˮ������ǣ�ԭ����
��ʼ�ų������Թ��ڵĿ���
��ʼ�ų������Թ��ڵĿ���
����2���������ȣ��۲쵽�Թܢ��г���ʯ��ˮ����ǣ��Թܢ��к�ɫ��ĩ�г��ֺ�ɫ���ʣ�����д�����е�һ����ѧ����ʽ��
C+2CuO
2Cu+CO2������CO2+Ca��OH��2�TCaCO3��+H2O��
| ||
C+2CuO
2Cu+CO2������CO2+Ca��OH��2�TCaCO3��+H2O��
��
| ||
��3����ַ�Ӧ�۲쵽�Թܢ��е�ʣ���������������������ɫ���壬Ϊ��ȷ���ú�ɫ����ijɷ֣�ͬѧ�ǽ���������̽������������������±���
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ������Ӧ����Թܢ��еĹ����ڽྻ�Թ��ڣ��� �� ϡ���� ϡ���� ���ȣ� |
������ | �ú�ɫ������ľ̿ |
��ɫ��ĩ�ܽ⣬��Һ����ɫ��Ϊ��ɫ ��ɫ��ĩ�ܽ⣬��Һ����ɫ��Ϊ��ɫ |
�ú�ɫ����������ͭ |
�ռ�β������ֹһ����̼�Դ�������Ⱦ
�ռ�β������ֹһ����̼�Դ�������Ⱦ
���Թܢ۵�������������
������
����������1�������Թ����п���������ʱ�����ͷ�����
��2������̼��������ͭ��Ӧ����ͭ�Ͷ�����̼��������̼��ʹ�����ʯ��ˮ����ǣ�д����Ӧ�ķ���ʽ��
��3������ľ̿������ͭ������ķ�Ӧ������
��4������һ����̼�ǶԴ�������Ⱦ����������������Թܢۿ�����Ч�ط�ֹ������
��2������̼��������ͭ��Ӧ����ͭ�Ͷ�����̼��������̼��ʹ�����ʯ��ˮ����ǣ�д����Ӧ�ķ���ʽ��
��3������ľ̿������ͭ������ķ�Ӧ������
��4������һ����̼�ǶԴ�������Ⱦ����������������Թܢۿ�����Ч�ط�ֹ������
����⣺��1�������Թ����п���������ʱ�����ͣ����ԣ���ʼ����ʱ�ų���ʱ���Թ��еĿ�����
��2���ڸ���ʱ��̼��������ͭ��Ӧ����ͭ�Ͷ�����̼��������̼�����������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ӧ�ķ���ʽ�ǣ�C+2CuO
2Cu+CO2����CO2+Ca��OH��2�TCaCO3��+H2O��
��3������ľ̿����������ķ�Ӧ������ͭ������ķ�Ӧ�����ԣ�Ϊ��ȷ���ú�ɫ����ijɷ֣�ͬѧ�ǽ���������̽���Ϊ��ȡ������Ӧ����Թܢ��еĹ����ڽྻ�Թ��ڣ�����ϡ���ᣬ�ȣ�����������ú�ɫ������ľ̿������ɫ��ĩ�ܽ⣬��Һ����ɫ��Ϊ��ɫ����ú�ɫ����������ͭ��
��4�������ڸ���ʱ��̼��������ͭ��Ӧ�л����һ����̼��һ����̼�Դ�������Ⱦ�����ԣ�ͼ��������������ǣ��ռ�β������ֹһ����̼�Դ�������Ⱦ�������Թܢۿ�����Ч�ط�ֹ��������ֹ�Թ�ը�ѣ�
�ʴ�Ϊ����1����ʼ�ų������Թ��ڵĿ�������2��C+2CuO
2Cu+CO2������CO2+Ca��OH��2�TCaCO3��+H2O������3��ϡ���ᣬ��ɫ��ĩ�ܽ⣬��Һ����ɫ��Ϊ��ɫ����4���ռ�β������ֹһ����̼�Դ�������Ⱦ����������
��2���ڸ���ʱ��̼��������ͭ��Ӧ����ͭ�Ͷ�����̼��������̼�����������Ʒ�Ӧ����̼��Ƴ�����ˮ����Ӧ�ķ���ʽ�ǣ�C+2CuO
| ||
��3������ľ̿����������ķ�Ӧ������ͭ������ķ�Ӧ�����ԣ�Ϊ��ȷ���ú�ɫ����ijɷ֣�ͬѧ�ǽ���������̽���Ϊ��ȡ������Ӧ����Թܢ��еĹ����ڽྻ�Թ��ڣ�����ϡ���ᣬ�ȣ�����������ú�ɫ������ľ̿������ɫ��ĩ�ܽ⣬��Һ����ɫ��Ϊ��ɫ����ú�ɫ����������ͭ��
��4�������ڸ���ʱ��̼��������ͭ��Ӧ�л����һ����̼��һ����̼�Դ�������Ⱦ�����ԣ�ͼ��������������ǣ��ռ�β������ֹһ����̼�Դ�������Ⱦ�������Թܢۿ�����Ч�ط�ֹ��������ֹ�Թ�ը�ѣ�
�ʴ�Ϊ����1����ʼ�ų������Թ��ڵĿ�������2��C+2CuO
| ||
�����������Ƕ�̼��ԭ����ͭ��ʵ���ʵ��������ۺϿ��飬ע������֪ʶ��Ǩ�ƺ�������þ��ܽϿ����⣮

��ϰ��ϵ�д�
�����Ŀ
 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����


 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������