��Ŀ����
��2010?����������������ͬ��������Һ���ܶȲ���ͬ�����ձ����£�| �������� | 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | 90% | 98% |
| �ܶȣ�g/mL�� | 1.07 | 1.14 | 1.22 | 1.30 | 1.40 | 1.50 | 1.61 | 1.73 | 1.81 | 1.84 |
��1��10mL98%��Ũ�����к�gH2SO4��
��2����ʽ�������Ľ���п��������
��3�������������ݣ����Թ��ɳ�������Һ�������������ܶȵĹ�ϵ�ǣ�10mLˮ��10mL��������Ϊb%��������Һ��ϣ���Ϻ���Һ����������Ӧ������֮�䣮
���𰸡���������1�����ݶ��ձ����ɲ��98%��Ũ�����ܶ�Ϊ1.84g/mL���ɼ���10mL98%Ũ�������Һ��������Ȼ�������������=��Һ������×���ʵ���������98%�������Ũ����������������
��2������п�����ᷴӦ�Ļ�ѧ����ʽ��������������������ɼ���μӷ�Ӧ�Ľ���п��������
��3������������Һ����������10%��98%����������Ӧ�أ�������Һ���ܶ�Ҳ�ڲ��������ݼ�ˮϡ�ͺ���Һ����ɣ�������Һ�����������������㹫ʽ���г���Ϻ���Һ����������������b%��Һ�ܶ�����Һ��������b%�Ĺ�ϵʽ���ɹ�ϵʽ�ж�������Һ���������������Ŀ��ܷ�Χ��
����⣺��1�����ݶ��ձ���98%��Ũ������ܶ�Ϊ1.84g/mL��10mL98%Ũ�������������������=10mL×1.84g/mL×98%��18.0g
�ʴ�Ϊ��18.0��
��2����ȡ10mLϡ���������������=18g× =1.8g����10mLϡ������п��ȫ��Ӧ����п������Ϊx
=1.8g����10mLϡ������п��ȫ��Ӧ����п������Ϊx
Zn+H2SO4=ZnSO4+H2��
65 98
x 1.8g
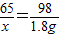 x��1.2g
x��1.2g
�����Ľ���п������ԼΪ1.2g��
��3���Ӷ��ձ����Եõ���������Һ����������10%��98%��������������Һ���ܶ�Ҳ�ڲ�������
�ʴ�Ϊ����������Խ���ܶ�Խ��
����������Һ���ܶ�Ϊ�ѣ�
10mLˮ��10mL��������Ϊb%��������Һ��ϣ���Ϻ���Һ����������=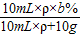 ×100%=
×100%=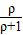 ×b%
×b%
�ٵ���=1.07g/mlʱ����Ϻ���Һ����������=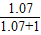 ×b%��0.52b%��
×b%��0.52b%��
�ڵ���=1.84g/mlʱ����Ϻ���Һ����������=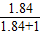 ×b%��0.65b%��
×b%��0.65b%��
���Ը���0.52b%��0.65b%��
�ʴ�Ϊ��0.52b%��0.65b%��
������������Һ��������������=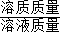 ×100%������������������֪���ɼ������������
×100%������������������֪���ɼ������������
��2������п�����ᷴӦ�Ļ�ѧ����ʽ��������������������ɼ���μӷ�Ӧ�Ľ���п��������
��3������������Һ����������10%��98%����������Ӧ�أ�������Һ���ܶ�Ҳ�ڲ��������ݼ�ˮϡ�ͺ���Һ����ɣ�������Һ�����������������㹫ʽ���г���Ϻ���Һ����������������b%��Һ�ܶ�����Һ��������b%�Ĺ�ϵʽ���ɹ�ϵʽ�ж�������Һ���������������Ŀ��ܷ�Χ��
����⣺��1�����ݶ��ձ���98%��Ũ������ܶ�Ϊ1.84g/mL��10mL98%Ũ�������������������=10mL×1.84g/mL×98%��18.0g
�ʴ�Ϊ��18.0��
��2����ȡ10mLϡ���������������=18g×
 =1.8g����10mLϡ������п��ȫ��Ӧ����п������Ϊx
=1.8g����10mLϡ������п��ȫ��Ӧ����п������ΪxZn+H2SO4=ZnSO4+H2��
65 98
x 1.8g
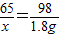 x��1.2g
x��1.2g�����Ľ���п������ԼΪ1.2g��
��3���Ӷ��ձ����Եõ���������Һ����������10%��98%��������������Һ���ܶ�Ҳ�ڲ�������
�ʴ�Ϊ����������Խ���ܶ�Խ��
����������Һ���ܶ�Ϊ�ѣ�
10mLˮ��10mL��������Ϊb%��������Һ��ϣ���Ϻ���Һ����������=
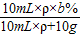 ×100%=
×100%=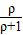 ×b%
×b%�ٵ���=1.07g/mlʱ����Ϻ���Һ����������=
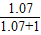 ×b%��0.52b%��
×b%��0.52b%���ڵ���=1.84g/mlʱ����Ϻ���Һ����������=
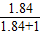 ×b%��0.65b%��
×b%��0.65b%�����Ը���0.52b%��0.65b%��
�ʴ�Ϊ��0.52b%��0.65b%��
������������Һ��������������=
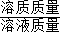 ×100%������������������֪���ɼ������������
×100%������������������֪���ɼ������������ 
��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
 2CO2+4H2O���ɴ˵ó������н��ۣ���ȫ��ȷ��һ���ǣ� ��
2CO2+4H2O���ɴ˵ó������н��ۣ���ȫ��ȷ��һ���ǣ� ��