��Ŀ����
����Ŀ���о���ѧϰ���⣺̽��ʵ�����о��õ�NaOH�ı��ʳ̶�
[�о�����]�ȳ�ȡ13.3g��NaOH��Ʒ(����ΪNa2CO3)�������Һ��Ȼ������Һ����μ�����������Ϊ14.6����ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȡ�
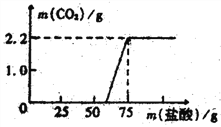
[�������]ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ��
��д�±���(����������С�������һλ)
Na2CO3������/g | _____________ |
����NaOH������/g | _____________ |
NaOH�ı��ʳ̶�(������������ʾ) | _____________ |
[����̽��]����ʵ���������NaOH��Ӧ�������������_____________��
[��������]��������NaOH��Ӧ���������������������ͼ���㷢����ʲô���⣿__________________________
���𰸡� 5.3 4 33.3% 50g ����������Һ��ȫ��Ӧ�����μ�HCl������������������̼����
���������⣺��ͼʾ��֪�����Ķ�����̼������Ϊ2.2g��
������2.2g������̼ʱ��Ҫ��̼����������x
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 2.2g
![]()
x=5.3g
����̼���Ƶ�����Ϊ5.3g���跴Ӧ���������Ƶ�����Ϊy
2NaOH+CO2�TNa2CO3+H2O
80 106
y 5.3g
![]()
=4g
���������Ƶı��ʳ̶�Ϊ![]() ��100%=33.3%��
��100%=33.3%��
[����̽��]û����������������Ϊ13.3g-5.3g=8g
��μӷ�Ӧ�Ȼ��������Ϊz
NaOH+HCl=NaCl+H2O
40 36.5
8g z
![]()
z=7.3g��
���������=![]() =50g��
=50g��
[��������]����ͼ�ο��Կ���NaOH����ȫ�кͺ�������Ϊ50g�����μ����ᣬΪʲôû����������CO2������

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ÿl00g������������ҪӪ���ɷֵ�ƽ������Ϊ
�ɷ� | ���� | ������ | ��֬ | ˮ | ά���� | �� | �� | п |
����/g | 0 | 16. 6 | 5.2 | 78.2 | 0.00004 | 0.038 | 0.0008 | 0.0009 |
��ò������ṩ��������������Ӫ������ ( )
A.������ B.����� C.�Ĵ�¦ D.������